Virus-specific antibody, in the absence of T cells, mediates demyelination in mice infected with a neurotropic coronavirus
- PMID: 15743792
- PMCID: PMC1602352
- DOI: 10.1016/S0002-9440(10)62301-2
Virus-specific antibody, in the absence of T cells, mediates demyelination in mice infected with a neurotropic coronavirus
Abstract
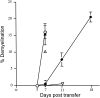
Mice infected with mouse hepatitis virus strain JHM develop an inflammatory demyelinating disease in the central nervous system with many similarities to human multiple sclerosis. The mouse disease is primarily immune-mediated because demyelination is not detected in JHM-infected mice lacking T or B cells but does occur after transfer of JHM-specific T cells. Although less is known about the ability of antibodies to mediate demyelination, the presence of oligoclonally expanded B cells and high concentrations of antibodies (against self or infectious agents) in the central nervous system of many multiple sclerosis patients suggests that antibodies may also contribute to myelin destruction. Here, we show that anti-JHM antibodies, in the absence of T or B cells, caused demyelination in JHM-infected mice. Anti-JHM antibody was detected adjacent to areas of demyelination, consistent with a direct interaction between antibody and infected cells. Demyelination was reduced by 85 to 90% in infected RAG1(-/-) mice lacking normal expression of activating Fc receptors (FcRgamma(-/-)) and by approximately 76% when complement was depleted by treatment with cobra venom factor. These data demonstrate that JHM-specific antibodies are sufficient to cause demyelination and that myelin destruction in the presence of anti-virus antibodies results from a combination of complement- and Fc receptor-dependent mechanisms.
Figures

Similar articles
-
Bystander CD4 T cells do not mediate demyelination in mice infected with a neurotropic coronavirus.J Neuroimmunol. 2003 Apr;137(1-2):42-50. doi: 10.1016/s0165-5728(03)00041-9. J Neuroimmunol. 2003. PMID: 12667646 Free PMC article.
-
CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination.J Immunol. 2000 Aug 15;165(4):2278-86. doi: 10.4049/jimmunol.165.4.2278. J Immunol. 2000. PMID: 10925317
-
Interaction of immune and central nervous systems: contribution of anti-viral Thy-1+ cells to demyelination induced by coronavirus JHM.Reg Immunol. 1993 Jan-Feb;5(1):37-43. Reg Immunol. 1993. PMID: 8102242
-
The role of myelin oligodendrocyte glycoprotein in autoimmune demyelination: a target for multiple sclerosis therapy?Expert Opin Ther Targets. 2012 May;16(5):451-62. doi: 10.1517/14728222.2012.677438. Epub 2012 Apr 12. Expert Opin Ther Targets. 2012. PMID: 22494461 Review.
-
Roles of regulatory T cells and IL-10 in virus-induced demyelination.J Neuroimmunol. 2017 Jul 15;308:6-11. doi: 10.1016/j.jneuroim.2017.01.001. Epub 2017 Jan 4. J Neuroimmunol. 2017. PMID: 28065579 Free PMC article. Review.
Cited by
-
Maturation and localization of macrophages and microglia during infection with a neurotropic murine coronavirus.Brain Pathol. 2008 Jan;18(1):40-51. doi: 10.1111/j.1750-3639.2007.00098.x. Epub 2007 Oct 12. Brain Pathol. 2008. PMID: 17935605 Free PMC article.
-
Immunopathogenesis of coronavirus infections: implications for SARS.Nat Rev Immunol. 2005 Dec;5(12):917-27. doi: 10.1038/nri1732. Nat Rev Immunol. 2005. PMID: 16322745 Free PMC article. Review.
-
Viral expression of CCL2 is sufficient to induce demyelination in RAG1-/- mice infected with a neurotropic coronavirus.J Virol. 2005 Jun;79(11):7113-20. doi: 10.1128/JVI.79.11.7113-7120.2005. J Virol. 2005. PMID: 15890951 Free PMC article.
-
Multiple sclerosis: experimental models and reality.Acta Neuropathol. 2017 Feb;133(2):223-244. doi: 10.1007/s00401-016-1631-4. Epub 2016 Oct 20. Acta Neuropathol. 2017. PMID: 27766432 Free PMC article. Review.
-
Coronaviruses post-SARS: update on replication and pathogenesis.Nat Rev Microbiol. 2009 Jun;7(6):439-50. doi: 10.1038/nrmicro2147. Nat Rev Microbiol. 2009. PMID: 19430490 Free PMC article. Review.
References
-
- Hemmer B, Archelos JJ, Hartung HP. New concepts in the immunopathogenesis of multiple sclerosis. Nat Rev Neurosci. 2002;3:291–301. - PubMed
-
- Noseworthy JH. Progress in determining the causes and treatment of multiple sclerosis. Nature. 1999;399:A40–A47. - PubMed
-
- Steinman L. Assessment of animal models for MS and demyelinating disease in the design of rational therapy. Neuron. 1999;24:511–514. - PubMed
-
- Wu GF, Dandekar AA, Pewe L, Perlman S. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J Immunol. 2000;165:2278–2286. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

