Complementarity in the supramolecular design of arenaviruses and retroviruses revealed by electron cryomicroscopy and image analysis
- PMID: 15731275
- PMCID: PMC1075687
- DOI: 10.1128/JVI.79.6.3822-3830.2005
Complementarity in the supramolecular design of arenaviruses and retroviruses revealed by electron cryomicroscopy and image analysis
Abstract
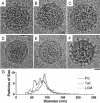
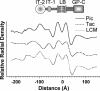
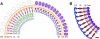
Arenaviruses are rodent-borne agents of diseases, including potentially lethal human hemorrhagic fevers. These enveloped viruses encapsidate a bisegmented ambisense single-stranded RNA genome that can be packaged in variable copy number. Electron cryomicroscopy and image analysis of New World Pichinde and Tacaribe arenaviruses and Old World lymphocytic choriomeningitis virus revealed pleomorphic enveloped particles ranging in diameter from approximately 400 to approximately 2,000 A. The surface spikes were spaced approximately 100 A apart and extended approximately 90 A from the maximum phospholipid headgroup density of the outer bilayer leaflet. Distinctive stalk and head regions extended radially approximately 30 and approximately 60 A from the outer bilayer leaflet, respectively. Two interior layers of density apposed to the inner leaflet of the viral lipid bilayer were assigned as protein Z and nucleoprotein (NP) molecules on the basis of their appearance, spacing, and projected volume. Analysis of en face views of virions lacking the GP-C spikes showed reflections consistent with paracrystalline packing of the NP molecules in a lattice with edges of approximately 57 and approximately 74 A. The structural proteins of retroviruses and arenaviruses assemble with similar radial density distributions, using common cellular components.
Figures



Similar articles
-
Multifunctional nature of the arenavirus RING finger protein Z.Viruses. 2012 Nov 9;4(11):2973-3011. doi: 10.3390/v4112973. Viruses. 2012. PMID: 23202512 Free PMC article. Review.
-
Characterization of the Lassa virus matrix protein Z: electron microscopic study of virus-like particles and interaction with the nucleoprotein (NP).Virus Res. 2004 Mar 15;100(2):249-55. doi: 10.1016/j.virusres.2003.11.017. Virus Res. 2004. PMID: 15019244
-
Cell entry by human pathogenic arenaviruses.Cell Microbiol. 2008 Apr;10(4):828-35. doi: 10.1111/j.1462-5822.2007.01113.x. Epub 2007 Dec 21. Cell Microbiol. 2008. PMID: 18182084 Review.
-
The Z proteins of pathogenic but not nonpathogenic arenaviruses inhibit RIG-I-like receptor-dependent interferon production.J Virol. 2015 Mar;89(5):2944-55. doi: 10.1128/JVI.03349-14. Epub 2014 Dec 31. J Virol. 2015. PMID: 25552708 Free PMC article.
-
Supramolecular architecture of severe acute respiratory syndrome coronavirus revealed by electron cryomicroscopy.J Virol. 2006 Aug;80(16):7918-28. doi: 10.1128/JVI.00645-06. J Virol. 2006. PMID: 16873249 Free PMC article.
Cited by
-
Architectural organization and in situ fusion protein structure of lymphocytic choriomeningitis virus.J Virol. 2024 Oct 22;98(10):e0064024. doi: 10.1128/jvi.00640-24. Epub 2024 Sep 27. J Virol. 2024. PMID: 39329471
-
Characterization of bi-segmented and tri-segmented recombinant Pichinde virus particles.J Virol. 2024 Oct 22;98(10):e0079924. doi: 10.1128/jvi.00799-24. Epub 2024 Sep 12. J Virol. 2024. PMID: 39264155
-
Treatment of highly virulent mammarenavirus infections-status quo and future directions.Expert Opin Drug Discov. 2024 May;19(5):537-551. doi: 10.1080/17460441.2024.2340494. Epub 2024 Apr 12. Expert Opin Drug Discov. 2024. PMID: 38606475 Review.
-
Negative and ambisense RNA virus ribonucleocapsids: more than protective armor.Microbiol Mol Biol Rev. 2023 Dec 20;87(4):e0008223. doi: 10.1128/mmbr.00082-23. Epub 2023 Sep 26. Microbiol Mol Biol Rev. 2023. PMID: 37750733 Free PMC article. Review.
-
Progress in Anti-Mammarenavirus Drug Development.Viruses. 2021 Jun 22;13(7):1187. doi: 10.3390/v13071187. Viruses. 2021. PMID: 34206216 Free PMC article. Review.
References
-
- Booy, F. P., R. W. H. Ruigrok, and E. F. J. van Bruggen. 1985. Electron microscopy of influenza virus. A comparison of negatively stained and ice-embedded particles. J. Mol. Biol. 184:667-676. - PubMed
-
- Bradford, M. M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72:248-254. - PubMed
-
- Burns, J. W., and M. J. Buchmeier. 1993. Glycoproteins of the arenaviruses, p. 17-35. In M. S. Salvato (ed.), The Arenaviridae. Plenum Press, New York, N.Y.
-
- Burns, J. W., and M. J. Buchmeier. 1991. Protein-protein interactions in lymphocytic choriomeningitis virus. Virology 183:620-629. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

