CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains
- PMID: 15729345
- PMCID: PMC2756053
- DOI: 10.1038/nature03313
CFTR channel opening by ATP-driven tight dimerization of its nucleotide-binding domains
Abstract
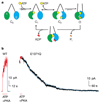
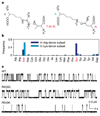
ABC (ATP-binding cassette) proteins constitute a large family of membrane proteins that actively transport a broad range of substrates. Cystic fibrosis transmembrane conductance regulator (CFTR), the protein dysfunctional in cystic fibrosis, is unique among ABC proteins in that its transmembrane domains comprise an ion channel. Opening and closing of the pore have been linked to ATP binding and hydrolysis at CFTR's two nucleotide-binding domains, NBD1 and NBD2 (see, for example, refs 1, 2). Isolated NBDs of prokaryotic ABC proteins dimerize upon binding ATP, and hydrolysis of the ATP causes dimer dissociation. Here, using single-channel recording methods on intact CFTR molecules, we directly follow opening and closing of the channel gates, and relate these occurrences to ATP-mediated events in the NBDs. We find that energetic coupling between two CFTR residues, expected to lie on opposite sides of its predicted NBD1-NBD2 dimer interface, changes in concert with channel gating status. The two monitored side chains are independent of each other in closed channels but become coupled as the channels open. The results directly link ATP-driven tight dimerization of CFTR's cytoplasmic nucleotide-binding domains to opening of the ion channel in the transmembrane domains. This establishes a molecular mechanism, involving dynamic restructuring of the NBD dimer interface, that is probably common to all members of the ABC protein superfamily.
Figures


Similar articles
-
Control of the CFTR channel's gates.Biochem Soc Trans. 2005 Nov;33(Pt 5):1003-7. doi: 10.1042/BST20051003. Biochem Soc Trans. 2005. PMID: 16246032 Free PMC article.
-
The two ATP binding sites of cystic fibrosis transmembrane conductance regulator (CFTR) play distinct roles in gating kinetics and energetics.J Gen Physiol. 2006 Oct;128(4):413-22. doi: 10.1085/jgp.200609622. Epub 2006 Sep 11. J Gen Physiol. 2006. PMID: 16966475 Free PMC article.
-
Prolonged nonhydrolytic interaction of nucleotide with CFTR's NH2-terminal nucleotide binding domain and its role in channel gating.J Gen Physiol. 2003 Sep;122(3):333-48. doi: 10.1085/jgp.200308798. J Gen Physiol. 2003. PMID: 12939393 Free PMC article.
-
Review. ATP hydrolysis-driven gating in cystic fibrosis transmembrane conductance regulator.Philos Trans R Soc Lond B Biol Sci. 2009 Jan 27;364(1514):247-55. doi: 10.1098/rstb.2008.0191. Philos Trans R Soc Lond B Biol Sci. 2009. PMID: 18957373 Free PMC article. Review.
-
ATP hydrolysis cycles and the gating of CFTR Cl- channels.Acta Physiol Scand Suppl. 1998 Aug;643:247-56. Acta Physiol Scand Suppl. 1998. PMID: 9789567 Review.
Cited by
-
Cysteine accessibility probes timing and extent of NBD separation along the dimer interface in gating CFTR channels.J Gen Physiol. 2015 Apr;145(4):261-83. doi: 10.1085/jgp.201411347. J Gen Physiol. 2015. PMID: 25825169 Free PMC article.
-
Structural identification of a selectivity filter in CFTR.Proc Natl Acad Sci U S A. 2024 Feb 27;121(9):e2316673121. doi: 10.1073/pnas.2316673121. Epub 2024 Feb 21. Proc Natl Acad Sci U S A. 2024. PMID: 38381791 Free PMC article.
-
G551D mutation impairs PKA-dependent activation of CFTR channel that can be restored by novel GOF mutations.Am J Physiol Lung Cell Mol Physiol. 2020 Nov 1;319(5):L770-L785. doi: 10.1152/ajplung.00262.2019. Epub 2020 Sep 2. Am J Physiol Lung Cell Mol Physiol. 2020. PMID: 32877225 Free PMC article.
-
Functional characterization reveals that zebrafish CFTR prefers to occupy closed channel conformations.PLoS One. 2018 Dec 31;13(12):e0209862. doi: 10.1371/journal.pone.0209862. eCollection 2018. PLoS One. 2018. PMID: 30596737 Free PMC article.
-
Role of CFTR's intrinsic adenylate kinase activity in gating of the Cl(-) channel.J Bioenerg Biomembr. 2007 Dec;39(5-6):473-9. doi: 10.1007/s10863-007-9119-5. J Bioenerg Biomembr. 2007. PMID: 17965924 Review.
References
-
- Gunderson KL, Kopito RR. Conformational states of CFTR associated with channel gating: the role of ATP binding and hydrolysis. Cell. 1995;82:231–239. - PubMed
-
- Carson MR, Travis SM, Welsh MJ. The two nucleotide-binding domains of cystic fibrosis transmembrane conductance regulator (CFTR) have distinct functions in controlling channel activity. J. Biol. Chem. 1995;270:1711–1717. - PubMed
-
- Hopfner KP, et al. Structural biology of Rad50 ATPase: ATP-driven conformational control in DNA double-strand break repair and the ABC-ATPase superfamily. Cell. 2000;101:789–800. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

