A Dictyostelium homologue of WASP is required for polarized F-actin assembly during chemotaxis
- PMID: 15728724
- PMCID: PMC1087228
- DOI: 10.1091/mbc.e04-09-0844
A Dictyostelium homologue of WASP is required for polarized F-actin assembly during chemotaxis
Abstract
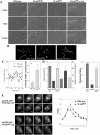
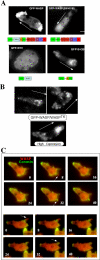
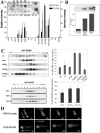
The actin cytoskeleton controls the overall structure of cells and is highly polarized in chemotaxing cells, with F-actin assembled predominantly in the anterior leading edge and to a lesser degree in the cell's posterior. Wiskott-Aldrich syndrome protein (WASP) has emerged as a central player in controlling actin polymerization. We have investigated WASP function and its regulation in chemotaxing Dictyostelium cells and demonstrated the specific and essential role of WASP in organizing polarized F-actin assembly in chemotaxing cells. Cells expressing very low levels of WASP show reduced F-actin levels and significant defects in polarized F-actin assembly, resulting in an inability to establish axial polarity during chemotaxis. GFP-WASP preferentially localizes at the leading edge and uropod of chemotaxing cells and the B domain of WASP is required for the localization of WASP. We demonstrated that the B domain binds to PI(4,5)P2 and PI(3,4,5)P3 with similar affinities. The interaction between the B domain and PI(3,4,5)P3 plays an important role for the localization of WASP to the leading edge in chemotaxing cells. Our results suggest that the spatial and temporal control of WASP localization and activation is essential for the regulation of directional motility.
Figures


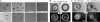

Similar articles
-
WASP-interacting protein is important for actin filament elongation and prompt pseudopod formation in response to a dynamic chemoattractant gradient.Mol Biol Cell. 2006 Oct;17(10):4564-75. doi: 10.1091/mbc.e05-10-0994. Epub 2006 Aug 9. Mol Biol Cell. 2006. PMID: 16899512 Free PMC article.
-
Role of RacC for the regulation of WASP and phosphatidylinositol 3-kinase during chemotaxis of Dictyostelium.J Biol Chem. 2006 Nov 17;281(46):35224-34. doi: 10.1074/jbc.M605997200. Epub 2006 Sep 12. J Biol Chem. 2006. PMID: 16968699 Free PMC article.
-
An attenuating role of a WASP-related protein, WASP-B, in the regulation of F-actin polymerization and pseudopod formation via the regulation of RacC during Dictyostelium chemotaxis.Biochem Biophys Res Commun. 2013 Jul 12;436(4):719-24. doi: 10.1016/j.bbrc.2013.06.022. Epub 2013 Jun 17. Biochem Biophys Res Commun. 2013. PMID: 23791739 Free PMC article.
-
The Wiskott-Aldrich syndrome protein: forging the link between actin and cell activation.Immunol Rev. 2003 Apr;192:98-112. doi: 10.1034/j.1600-065x.2003.00031.x. Immunol Rev. 2003. PMID: 12670398 Review.
-
Feedback signaling controls leading-edge formation during chemotaxis.Curr Opin Genet Dev. 2006 Aug;16(4):339-47. doi: 10.1016/j.gde.2006.06.016. Epub 2006 Jun 27. Curr Opin Genet Dev. 2006. PMID: 16806895 Review.
Cited by
-
A continuum model of actin waves in Dictyostelium discoideum.PLoS One. 2013 May 31;8(5):e64272. doi: 10.1371/journal.pone.0064272. Print 2013. PLoS One. 2013. PMID: 23741312 Free PMC article.
-
Why Does Synergistic Activation of WASP, but Not N-WASP, by Cdc42 and PIP2 Require Cdc42 Prenylation?J Mol Biol. 2023 Apr 15;435(8):168035. doi: 10.1016/j.jmb.2023.168035. Epub 2023 Feb 28. J Mol Biol. 2023. PMID: 36863659 Free PMC article.
-
WASP and SCAR are evolutionarily conserved in actin-filled pseudopod-based motility.J Cell Biol. 2017 Jun 5;216(6):1673-1688. doi: 10.1083/jcb.201701074. Epub 2017 May 4. J Cell Biol. 2017. PMID: 28473602 Free PMC article.
-
Impact of the carbazole derivative wiskostatin on mechanical stability and dynamics of motile cells.J Muscle Res Cell Motil. 2012 Jun;33(2):95-106. doi: 10.1007/s10974-012-9287-8. Epub 2012 Mar 11. J Muscle Res Cell Motil. 2012. PMID: 22407517
-
Ras, PI3K and mTORC2 - three's a crowd?J Cell Sci. 2020 Oct 8;133(19):jcs234930. doi: 10.1242/jcs.234930. J Cell Sci. 2020. PMID: 33033115 Free PMC article. Review.
References
-
- Aldrich, R. A., Steinberg, A. G., and Campbell, D. C. (1954). Pedigree demonstrating a sex linked recessive condition characterized by draining ears, eczematoid dermatitis, and bloody diarrhea. Pediatrics 13, 133–139. - PubMed
-
- Asano, S., Mishima, M., and Nishida, E. (2001). Coronin forms a stable dimer through its C-terminal coiled coil region: an implicated role in its localization to cell periphery. Genes Cells 6, 225–235. - PubMed
-
- Aubry, L., and Firtel, R. (1999). Integration of signaling networks that regulate Dictyostelium differentiation. Annu. Rev. Cell Dev. Biol. 15, 469–4517. - PubMed
-
- Badolato, R., Sozzani, S., Malacarne, F., Bresciani, S., Fiorini, M., Borsatti, A., Albertini, A., Mantovani, A., Ugazio, A. G., and Notarangelo, L. D. (1998). Monocytes from Wiskott-Aldrich patients display reduced chemotaxis and lack of cell polarization in response to monocyte chemoattractant protein-1 and formyl-methionyl-leucyl-phenylalanine. J. Immunol. 161, 1026–1033. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

