Small-scale molecular motions accomplish glutamate uptake in human glutamate transporters
- PMID: 15716409
- PMCID: PMC6725926
- DOI: 10.1523/JNEUROSCI.4138-04.2005
Small-scale molecular motions accomplish glutamate uptake in human glutamate transporters
Abstract
Glutamate transporters remove glutamate from the synaptic cleft to maintain efficient synaptic communication between neurons and to prevent glutamate concentrations from reaching neurotoxic levels. Glutamate transporters play an important role in ischemic neuronal death during stroke and have been implicated in epilepsy and amytropic lateral sclerosis. However, the molecular structure and the glutamate-uptake mechanism of these transporters are not well understood. The most recent models of glutamate transporters have three or five subunits, each with eight transmembrane domains, and one or two membrane-inserted loops. Here, using fluorescence resonance energy transfer (FRET) analysis, we have determined the relative position of the extracellular regions of these domains. Our results are consistent with a trimeric glutamate transporter with a large (>45 A) extracellular vestibule. In contrast to other transport proteins, our FRET measurements indicate that there are no large-scale motions in glutamate transporters and that glutamate uptake is accompanied by relatively small motions around the glutamate-binding sites. The large extracellular vestibule and the small-scale conformational changes could contribute to the fast kinetics predicted for glutamate transporters. Furthermore, we show that, despite the multimeric nature of glutamate transporters, the subunits function independently.
Figures

 ). D, Fluorescence labeling curve for A430C (•) and V273C (□) labeled with Alexa Fluor 488 C5-maleimide. Arrows indicate the amount of labeling used in the intrasubunit experiments. E, Fluorescence emission from an oocyte expressing 334C EAAT3 transporters labeled with only donor fluorophores (▪) and donor and acceptor fluorophores (▵). FRET efficiency is estimated as E = 1 - FDA/FD (Selvin, 1995). Endogenous fluorescence from an uninjected, labeled oocyte is also shown (•).
). D, Fluorescence labeling curve for A430C (•) and V273C (□) labeled with Alexa Fluor 488 C5-maleimide. Arrows indicate the amount of labeling used in the intrasubunit experiments. E, Fluorescence emission from an oocyte expressing 334C EAAT3 transporters labeled with only donor fluorophores (▪) and donor and acceptor fluorophores (▵). FRET efficiency is estimated as E = 1 - FDA/FD (Selvin, 1995). Endogenous fluorescence from an uninjected, labeled oocyte is also shown (•).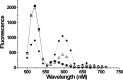
Similar articles
-
Fluorometric measurements of conformational changes in glutamate transporters.Proc Natl Acad Sci U S A. 2004 Mar 16;101(11):3951-6. doi: 10.1073/pnas.0306737101. Epub 2004 Mar 4. Proc Natl Acad Sci U S A. 2004. PMID: 15001707 Free PMC article.
-
Voltage-independent sodium-binding events reported by the 4B-4C loop in the human glutamate transporter excitatory amino acid transporter 3.J Biol Chem. 2007 Aug 24;282(34):24547-53. doi: 10.1074/jbc.M704087200. Epub 2007 Jun 22. J Biol Chem. 2007. PMID: 17588938
-
Functional significance of N- and C-terminus of the amino acid transporters EAAC1 and ASCT1: characterization of chimeric transporters.Biochim Biophys Acta. 2000 Aug 25;1467(2):338-46. doi: 10.1016/s0005-2736(00)00232-7. Biochim Biophys Acta. 2000. PMID: 11030592
-
Electrogenic glutamate transporters in the CNS: molecular mechanism, pre-steady-state kinetics, and their impact on synaptic signaling.J Membr Biol. 2005 Jan;203(1):1-20. doi: 10.1007/s00232-004-0731-6. J Membr Biol. 2005. PMID: 15834685 Free PMC article. Review.
-
The dual-function glutamate transporters: structure and molecular characterisation of the substrate-binding sites.Biochim Biophys Acta. 2002 Sep 10;1555(1-3):92-5. doi: 10.1016/s0005-2728(02)00260-8. Biochim Biophys Acta. 2002. PMID: 12206897 Review.
Cited by
-
Conformational ensemble of the sodium-coupled aspartate transporter.Nat Struct Mol Biol. 2013 Feb;20(2):215-21. doi: 10.1038/nsmb.2494. Epub 2013 Jan 20. Nat Struct Mol Biol. 2013. PMID: 23334289 Free PMC article.
-
Biochemical characterization of the C4-dicarboxylate transporter DctA from Bacillus subtilis.J Bacteriol. 2010 Jun;192(11):2900-7. doi: 10.1128/JB.00136-10. Epub 2010 Apr 2. J Bacteriol. 2010. PMID: 20363944 Free PMC article.
-
Appraising the Role of Astrocytes as Suppliers of Neuronal Glutathione Precursors.Int J Mol Sci. 2023 Apr 29;24(9):8059. doi: 10.3390/ijms24098059. Int J Mol Sci. 2023. PMID: 37175763 Free PMC article. Review.
-
Capturing Functional Motions of Membrane Channels and Transporters with Molecular Dynamics Simulation.J Comput Theor Nanosci. 2010 Dec;7(12):2481-2500. doi: 10.1166/jctn.2010.1636. J Comput Theor Nanosci. 2010. PMID: 23710155 Free PMC article.
-
A conserved methionine residue controls the substrate selectivity of a neuronal glutamate transporter.J Biol Chem. 2010 Jul 9;285(28):21241-8. doi: 10.1074/jbc.M109.087163. Epub 2010 Apr 27. J Biol Chem. 2010. PMID: 20424168 Free PMC article.
References
-
- Abramson J, Smirnova I, Kasho V, Verner G, Kaback HR, Iwata S (2003) Structure and mechanism of the lactose permease of Escherichia coli Science 301: 610-615. - PubMed
-
- Bergles DE, Diamond JS, Jahr CE (1999) Clearance of glutamate inside the synapse and beyond. Curr Opin Neurobiol 9: 293-298. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
