Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection
- PMID: 15709010
- PMCID: PMC548446
- DOI: 10.1128/JVI.79.5.2910-2919.2005
Synthetic double-stranded RNA poly(I:C) combined with mucosal vaccine protects against influenza virus infection
Abstract
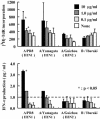
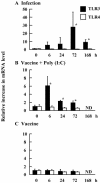
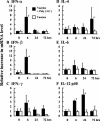
The mucosal adjuvant effect of synthetic double-stranded RNA polyriboinosinic polyribocytidylic acid [poly(I:C)] against influenza virus was examined under intranasal coadministration with inactivated hemagglutinin (HA) vaccine in BALB/c mice and was shown to have a protective effect against both nasal-restricted infection and lethal lung infection. Intranasal administration of vaccine from PR8 (H1N1) with poly(I:C) induced a high anti-HA immunoglobulin A (IgA) response in the nasal wash and IgG antibody response in the serum, while vaccination without poly(I:C) induced little response. Intracerebral injection confirmed the safety of poly(I:C). In addition, we demonstrated that administration of poly(I:C) with either A/Beijing (H1N1) or A/Yamagata (H1N1) vaccine conferred complete protection against PR8 challenge in this mouse nasal infection model, suggesting that poly(I:C) possessed cross-protection ability against variant viruses. To investigate the mechanism of the protective effect of poly(I:C), mRNA levels of Toll-like receptors and cytokines were examined in the nasal-associated lymphoid tissue after vaccination or virus challenge. Intranasal administration of HA vaccine with poly(I:C) up-regulated expression of Toll-like receptor 3 and alpha/beta interferons as well as Th1- and Th2-related cytokines. We propose that poly(I:C) is a new effective intranasal adjuvant for influenza virus vaccine.
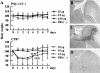
Figures
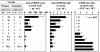



Similar articles
-
Protection against influenza virus infection by intranasal vaccine with surf clam microparticles (SMP) as an adjuvant.J Med Virol. 2006 Jul;78(7):954-63. doi: 10.1002/jmv.20647. J Med Virol. 2006. PMID: 16721854
-
Induction of cross-protective immunity against influenza A virus H5N1 by an intranasal vaccine with extracts of mushroom mycelia.J Med Virol. 2010 Jan;82(1):128-37. doi: 10.1002/jmv.21670. J Med Virol. 2010. PMID: 19950232
-
Protection against influenza virus infection by intranasal administration of hemagglutinin vaccine with chitin microparticles as an adjuvant.J Med Virol. 2005 Jan;75(1):130-6. doi: 10.1002/jmv.20247. J Med Virol. 2005. PMID: 15543590
-
Studies on the usefulness of intranasal inactivated influenza vaccines.Vaccine. 2010 Aug 31;28(38):6393-7. doi: 10.1016/j.vaccine.2010.05.019. Epub 2010 May 20. Vaccine. 2010. PMID: 20493820 Review.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
Cited by
-
A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge.Proc Natl Acad Sci U S A. 2011 Dec 20;108(51):20695-700. doi: 10.1073/pnas.1117715108. Epub 2011 Dec 5. Proc Natl Acad Sci U S A. 2011. PMID: 22143779 Free PMC article.
-
Variable deficiencies in the interferon response enhance susceptibility to vesicular stomatitis virus oncolytic actions in glioblastoma cells but not in normal human glial cells.J Virol. 2007 Feb;81(3):1479-91. doi: 10.1128/JVI.01861-06. Epub 2006 Nov 15. J Virol. 2007. PMID: 17108037 Free PMC article.
-
The effect of Toll-like receptor agonists on the immunogenicity of MVA-SARS-2-S vaccine after intranasal administration in mice.Front Cell Infect Microbiol. 2023 Oct 3;13:1259822. doi: 10.3389/fcimb.2023.1259822. eCollection 2023. Front Cell Infect Microbiol. 2023. PMID: 37854858 Free PMC article.
-
Application of spatial transcriptomics analysis using the Visium system for the mouse nasal cavity after intranasal vaccination.Front Immunol. 2023 Jul 21;14:1209945. doi: 10.3389/fimmu.2023.1209945. eCollection 2023. Front Immunol. 2023. PMID: 37545501 Free PMC article.
-
Mucosal polyinosinic-polycytidylic acid improves protection elicited by replicating influenza vaccines via enhanced dendritic cell function and T cell immunity.J Immunol. 2014 Aug 1;193(3):1324-32. doi: 10.4049/jimmunol.1400222. Epub 2014 Jun 23. J Immunol. 2014. PMID: 24958904 Free PMC article.
References
-
- Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. - PubMed
-
- Asahi, Y., T. Yoshikawa, I. Watanabe, T. Iwasaki, H. Hasegawa, Y. Sato, S. Shimada, M. Nanno, Y. Matsuoka, M. Ohwaki, Y. Iwakura, Y. Suzuki, C. Aizawa, T. Sata, T. Kurata, and S. Tamura. 2002. Protection against influenza virus infection in polymeric Ig receptor knockout mice immunized intranasally with adjuvant-combined vaccines. J. Immunol. 168:2930-2938. - PubMed
-
- Couch, R. B., and J. A. Kasel. 1983. Immunity to influenza in man. Annu. Rev. Microbiol. 37:529-549. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

