Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice
- PMID: 15703389
- PMCID: PMC6725991
- DOI: 10.1523/JNEUROSCI.3034-04.2005
Myelinogenesis and axonal recognition by oligodendrocytes in brain are uncoupled in Olig1-null mice
Abstract
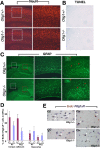
Myelin-forming oligodendrocytes facilitate saltatory nerve conduction and support neuronal functions in the mammalian CNS. Although the processes of oligodendrogliogenesis and differentiation from neural progenitor cells have come to light in recent years, the molecular mechanisms underlying oligodendrocyte myelinogenesis are poorly defined. Herein, we demonstrate the pivotal role of the basic helix-loop-helix transcription factor, Olig1, in oligodendrocyte myelinogenesis in brain development. Mice lacking a functional Olig1 gene develop severe neurological deficits and die in the third postnatal week. In the brains of these mice, expression of myelin-specific genes is abolished, whereas the formation of oligodendrocyte progenitors is not affected. Furthermore, multilamellar wrapping of myelin membranes around axons does not occur, despite recognition and contact of axons by oligodendrocytes, and Olig1-null mice develop widespread progressive axonal degeneration and gliosis. In contrast, myelin sheaths are formed in the spinal cord, although the extent of myelination is severely reduced. At the molecular level, we find that Olig1 regulates transcription of the major myelin-specific genes, Mbp, Plp1, and Mag, and suppresses expression of a major astrocyte-specific gene, Gfap. Together, our data indicate that Olig1 is a central regulator of oligodendrocyte myelinogenesis in brain and that axonal recognition and myelination by oligodendrocytes are separable processes.
Figures



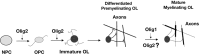
Similar articles
-
Cyclin dependent kinase 5 is required for the normal development of oligodendrocytes and myelin formation.Dev Biol. 2013 Jun 15;378(2):94-106. doi: 10.1016/j.ydbio.2013.03.023. Epub 2013 Apr 10. Dev Biol. 2013. PMID: 23583582 Free PMC article.
-
Olig1 function is required for oligodendrocyte differentiation in the mouse brain.J Neurosci. 2015 Mar 11;35(10):4386-402. doi: 10.1523/JNEUROSCI.4962-14.2015. J Neurosci. 2015. PMID: 25762682 Free PMC article.
-
Effects of progesterone on oligodendrocyte progenitors, oligodendrocyte transcription factors, and myelin proteins following spinal cord injury.Glia. 2009 Jun;57(8):884-97. doi: 10.1002/glia.20814. Glia. 2009. PMID: 19053058
-
Oligodendrocytes and the control of myelination in vivo: new insights from the rat anterior medullary velum.J Neurosci Res. 2000 Feb 15;59(4):477-88. doi: 10.1002/(SICI)1097-4547(20000215)59:4<477::AID-JNR2>3.0.CO;2-J. J Neurosci Res. 2000. PMID: 10679786 Review.
-
Mechanisms regulating the development of oligodendrocytes and central nervous system myelin.Neuroscience. 2014 Sep 12;276:29-47. doi: 10.1016/j.neuroscience.2013.11.029. Epub 2013 Nov 22. Neuroscience. 2014. PMID: 24275321 Review.
Cited by
-
Fine-Tuning Oligodendrocyte Development by microRNAs.Front Neurosci. 2012 Feb 6;6:13. doi: 10.3389/fnins.2012.00013. eCollection 2012. Front Neurosci. 2012. PMID: 22347159 Free PMC article.
-
Stem cells, progenitors and myelin repair.J Anat. 2005 Sep;207(3):251-8. doi: 10.1111/j.1469-7580.2005.00456.x. J Anat. 2005. PMID: 16185249 Free PMC article. Review.
-
ASCL1 regulates proliferation of NG2-glia in the embryonic and adult spinal cord.Glia. 2018 Sep;66(9):1862-1880. doi: 10.1002/glia.23344. Epub 2018 Apr 23. Glia. 2018. PMID: 29683222 Free PMC article.
-
Evolution of the CNS myelin gene regulatory program.Brain Res. 2016 Jun 15;1641(Pt A):111-121. doi: 10.1016/j.brainres.2015.10.013. Epub 2015 Oct 22. Brain Res. 2016. PMID: 26474911 Free PMC article. Review.
-
GPR17 gene disruption does not alter food intake or glucose homeostasis in mice.Proc Natl Acad Sci U S A. 2015 Feb 10;112(6):1845-9. doi: 10.1073/pnas.1424968112. Epub 2015 Jan 26. Proc Natl Acad Sci U S A. 2015. PMID: 25624481 Free PMC article.
References
-
- Barres BA, Raff MC (1994) Control of oligodendrocyte number in the developing rat optic nerve. Neuron 12: 935-942. - PubMed
-
- Barres BA, Schmid R, Sendnter M, Raff MC (1993) Multiple extracellular signals are required for long-term oligodendrocyte survival. Development 118: 283-295. - PubMed
-
- Barres BA, Lazar MA, Raff MC (1994) A novel role for thyroid hormone, glucocorticoids and retinoic acid in timing oligodendrocyte development. Development 120: 1097-1108. - PubMed
-
- Berger J, Moser HW, Forss-Petter S (2001) Leukodystrophies: recent developments in genetics, molecular biology, pathogenesis and treatment. Curr Opin Neurol 14: 305-312. - PubMed
-
- Bhat RV, Axt KJ, Fosnaugh JS, Smith KJ, Johnson KA, Hill DE, Kinzler KW, Baraban JM (1996) Expression of the APC tumor suppressor protein in oligodendroglia. Glia 17: 169-174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous
