Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand
- PMID: 15701701
- PMCID: PMC548962
- DOI: 10.1073/pnas.0408384102
Nitrolinoleic acid: an endogenous peroxisome proliferator-activated receptor gamma ligand
Abstract
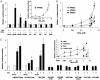
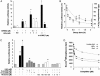
Nitroalkene derivatives of linoleic acid (nitrolinoleic acid, LNO2) are formed via nitric oxide-dependent oxidative inflammatory reactions and are found at concentrations of approximately 500 nM in the blood of healthy individuals. We report that LNO2 is a potent endogenous ligand for peroxisome proliferator-activated receptor gamma (PPARgamma; Ki approximately 133 nM) that acts within physiological concentration ranges. This nuclear hormone receptor (PPARgamma) regulates glucose homeostasis, lipid metabolism, and inflammation. PPARgamma ligand activity is specific for LNO2)and not mediated by LNO2 decay products, NO donors, linoleic acid (LA), or oxidized LA. LNO2 is a significantly more robust PPARgamma ligand than other reported endogenous PPARgamma ligands, including lysophosphatidic acid (16:0 and 18:1), 15-deoxy-Delta12,14-PGJ2, conjugated LA and azelaoyl-phosphocholine. LNO2 activation of PPARgamma via CV-1 cell luciferase reporter gene expression analysis revealed a ligand activity that rivals or exceeds synthetic PPARgamma agonists such as rosiglitazone and ciglitazone, is coactivated by 9 cis-retinoic acid and is inhibited by the PPARgamma antagonist GW9662. LNO2 induces PPARgamma-dependent macrophage CD-36 expression, adipocyte differentiation, and glucose uptake also at a potency rivaling thiazolidinediones. These observations reveal that NO-mediated cell signaling reactions can be transduced by fatty acid nitration products and PPAR-dependent gene expression.
Figures
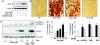
Similar articles
-
Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor gamma-dependent mechanisms.Arterioscler Thromb Vasc Biol. 2005 Sep;25(9):1810-6. doi: 10.1161/01.ATV.0000177805.65864.d4. Epub 2005 Jul 14. Arterioscler Thromb Vasc Biol. 2005. PMID: 16020752
-
Fatty acid transduction of nitric oxide signaling: multiple nitrated unsaturated fatty acid derivatives exist in human blood and urine and serve as endogenous peroxisome proliferator-activated receptor ligands.J Biol Chem. 2005 Dec 23;280(51):42464-75. doi: 10.1074/jbc.M504212200. Epub 2005 Oct 14. J Biol Chem. 2005. PMID: 16227625 Free PMC article.
-
Peroxisome proliferator-activated receptor gamma is required for the inhibitory effect of ciglitazone but not 15-deoxy-Delta 12,14-prostaglandin J2 on the NFkappaB pathway in human endothelial cells.Shock. 2007 Dec;28(6):722-726. doi: 10.1097/SHK.0b013e318055683a. Shock. 2007. PMID: 17621259
-
Peroxisome proliferator-activated receptor-gamma is a new therapeutic target in sepsis and inflammation.Shock. 2005 May;23(5):393-9. doi: 10.1097/01.shk.0000160521.91363.88. Shock. 2005. PMID: 15834303 Review.
-
PPARγ signaling and emerging opportunities for improved therapeutics.Pharmacol Res. 2016 Sep;111:76-85. doi: 10.1016/j.phrs.2016.02.028. Epub 2016 Jun 4. Pharmacol Res. 2016. PMID: 27268145 Free PMC article. Review.
Cited by
-
The Versatile Role of Peroxisome Proliferator-Activated Receptors in Immune-Mediated Intestinal Diseases.Cells. 2024 Oct 12;13(20):1688. doi: 10.3390/cells13201688. Cells. 2024. PMID: 39451206 Free PMC article. Review.
-
Thioredoxin reductase 1 suppresses adipocyte differentiation and insulin responsiveness.Sci Rep. 2016 Jun 27;6:28080. doi: 10.1038/srep28080. Sci Rep. 2016. PMID: 27346647 Free PMC article.
-
Self-regulation of the inflammatory response by peroxisome proliferator-activated receptors.Inflamm Res. 2019 Jun;68(6):443-458. doi: 10.1007/s00011-019-01231-1. Epub 2019 Mar 29. Inflamm Res. 2019. PMID: 30927048 Free PMC article. Review.
-
Electrophilic nitro-fatty acids activate NRF2 by a KEAP1 cysteine 151-independent mechanism.J Biol Chem. 2011 Apr 22;286(16):14019-27. doi: 10.1074/jbc.M110.190710. Epub 2011 Feb 25. J Biol Chem. 2011. PMID: 21357422 Free PMC article.
-
Combined losartan and nitro-oleic acid remarkably improves diabetic nephropathy in mice.Am J Physiol Renal Physiol. 2013 Dec 1;305(11):F1555-62. doi: 10.1152/ajprenal.00157.2013. Epub 2013 Aug 14. Am J Physiol Renal Physiol. 2013. PMID: 23946292 Free PMC article.
References
-
- Schopfer, F. J., Baker, P. R. & Freeman, B. A. (2003) Trends Biochem. Sci. 28, 646-654. - PubMed
-
- Hogg, N. (2002) Annu. Rev. Pharmacol. Toxicol. 42, 585-600. - PubMed
-
- Rubbo, H., Radi, R., Anselmi, D., Kirk, M., Barnes, S., Butler, J., Eiserich, J. P. & Freeman, B. A. (2000) J. Biol. Chem. 275, 10812-10818. - PubMed
-
- Marnett, L. J., Wright, T. L., Crews, B. C., Tannenbaum, S. R. & Morrow, J. D. (2000) J. Biol. Chem. 275, 13427-13430. - PubMed
-
- O'Donnell, V. B. & Freeman, B. A. (2001) Circ. Res. 88, 12-21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HL03676/HL/NHLBI NIH HHS/United States
- R01 HL068878/HL/NHLBI NIH HHS/United States
- R37 HL058115/HL/NHLBI NIH HHS/United States
- S06GM08248/GM/NIGMS NIH HHS/United States
- R01 HL058115/HL/NHLBI NIH HHS/United States
- T32HL07457/HL/NHLBI NIH HHS/United States
- R25 HL003676/HL/NHLBI NIH HHS/United States
- T32 HL007457/HL/NHLBI NIH HHS/United States
- S06 GM008248/GM/NIGMS NIH HHS/United States
- HL64937/HL/NHLBI NIH HHS/United States
- UH1 HL003676/HL/NHLBI NIH HHS/United States
- R01 HL064937/HL/NHLBI NIH HHS/United States
- HL075397/HL/NHLBI NIH HHS/United States
- R01 HL075397/HL/NHLBI NIH HHS/United States
- HL58115/HL/NHLBI NIH HHS/United States
- HL068878/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

