DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo
- PMID: 15699213
- PMCID: PMC2171764
- DOI: 10.1083/jcb.200407124
DRhoGEF2 regulates actin organization and contractility in the Drosophila blastoderm embryo
Abstract
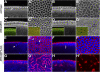
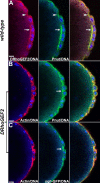
Morphogenesis of the Drosophila melanogaster embryo is associated with a dynamic reorganization of the actin cytoskeleton that is mediated by small GTPases of the Rho family. Often, Rho1 controls different aspects of cytoskeletal function in parallel, requiring a complex level of regulation. We show that the guanine triphosphate (GTP) exchange factor DRhoGEF2 is apically localized in epithelial cells throughout embryogenesis. We demonstrate that DRhoGEF2, which has previously been shown to regulate cell shape changes during gastrulation, recruits Rho1 to actin rings and regulates actin distribution and actomyosin contractility during nuclear divisions, pole cell formation, and cellularization of syncytial blastoderm embryos. We propose that DRhoGEF2 activity coordinates contractile actomyosin forces throughout morphogenesis in Drosophila by regulating the association of myosin with actin to form contractile cables. Our results support the hypothesis that specific aspects of Rho1 function are regulated by specific GTP exchange factors.
Figures



Similar articles
-
DRhoGEF2 and diaphanous regulate contractile force during segmental groove morphogenesis in the Drosophila embryo.Mol Biol Cell. 2008 May;19(5):1883-92. doi: 10.1091/mbc.e07-12-1230. Epub 2008 Feb 20. Mol Biol Cell. 2008. PMID: 18287521 Free PMC article.
-
An Arf-GEF regulates antagonism between endocytosis and the cytoskeleton for Drosophila blastoderm development.Curr Biol. 2013 Nov 4;23(21):2110-20. doi: 10.1016/j.cub.2013.08.058. Epub 2013 Oct 10. Curr Biol. 2013. PMID: 24120639
-
Cellularization in Drosophila melanogaster is disrupted by the inhibition of rho activity and the activation of Cdc42 function.Dev Biol. 1998 Dec 1;204(1):151-64. doi: 10.1006/dbio.1998.9061. Dev Biol. 1998. PMID: 9851849
-
How one becomes many: blastoderm cellularization in Drosophila melanogaster.Bioessays. 2002 Nov;24(11):1012-22. doi: 10.1002/bies.10184. Bioessays. 2002. PMID: 12386932 Review.
-
The cytoskeleton and morphogenesis of the early Drosophila embryo.Curr Opin Cell Biol. 1995 Feb;7(1):18-22. doi: 10.1016/0955-0674(95)80040-9. Curr Opin Cell Biol. 1995. PMID: 7755985 Review.
Cited by
-
The drosophila fragile X protein dFMR1 is required during early embryogenesis for pole cell formation and rapid nuclear division cycles.Genetics. 2006 Nov;174(3):1287-98. doi: 10.1534/genetics.106.062414. Epub 2006 Aug 3. Genetics. 2006. PMID: 16888325 Free PMC article.
-
A novel role for an APC2-Diaphanous complex in regulating actin organization in Drosophila.Development. 2009 Apr;136(8):1283-93. doi: 10.1242/dev.026963. Epub 2009 Mar 11. Development. 2009. PMID: 19279137 Free PMC article.
-
RhoGEF and positioning of rappaport-like furrows in the early Drosophila embryo.Curr Biol. 2012 Nov 6;22(21):2037-41. doi: 10.1016/j.cub.2012.08.046. Epub 2012 Sep 27. Curr Biol. 2012. PMID: 23022066 Free PMC article.
-
Pseudocleavage furrows restrict plasma membrane-associated PH domain in syncytial Drosophila embryos.Biophys J. 2022 Jun 21;121(12):2419-2435. doi: 10.1016/j.bpj.2022.05.015. Epub 2022 May 18. Biophys J. 2022. PMID: 35591789 Free PMC article.
-
Origin and development of primary animal epithelia.Bioessays. 2024 Feb;46(2):e2300150. doi: 10.1002/bies.202300150. Epub 2023 Nov 27. Bioessays. 2024. PMID: 38009581 Review.
References
-
- Afshar, K., B. Stuart, and S.A. Wasserman. 2000. Functional analysis of the Drosophila diaphanous FH protein in early embryonic development. Development. 127:1887–1897. - PubMed
-
- Barrett, K., M. Leptin, and J. Settleman. 1997. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophila gastrulation. Cell. 91:905–915. - PubMed
-
- Bate, M., and A. Martinez Arias. 1993. The Development of Drosophila melanogaster. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 1558.
-
- Brand, A.H., and N. Perrimon. 1993. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 118:401–415. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

