PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis
- PMID: 15692567
- PMCID: PMC549619
- DOI: 10.1038/sj.emboj.7600559
PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis
Erratum in
- EMBO J. 2005 Mar 23;24(6):1301
Abstract
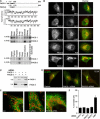
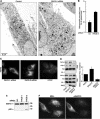
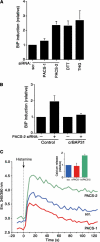
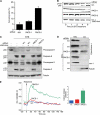
The endoplasmic reticulum (ER) and mitochondria form contacts that support communication between these two organelles, including synthesis and transfer of lipids, and the exchange of calcium, which regulates ER chaperones, mitochondrial ATP production, and apoptosis. Despite the fundamental roles for ER-mitochondria contacts, little is known about the molecules that regulate them. Here we report the identification of a multifunctional sorting protein, PACS-2, that integrates ER-mitochondria communication, ER homeostasis, and apoptosis. PACS-2 controls the apposition of mitochondria with the ER, as depletion of PACS-2 causes BAP31-dependent mitochondria fragmentation and uncoupling from the ER. PACS-2 also controls formation of ER lipid-synthesizing centers found on mitochondria-associated membranes and ER homeostasis. However, in response to apoptotic inducers, PACS-2 translocates Bid to mitochondria, which initiates a sequence of events including the formation of mitochondrial truncated Bid, the release of cytochrome c, and the activation of caspase-3, thereby causing cell death. Together, our results identify PACS-2 as a novel sorting protein that links the ER-mitochondria axis to ER homeostasis and the control of cell fate, and provide new insights into Bid action.
Figures
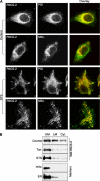
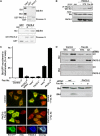
Similar articles
-
Translocation of Bax and Bid to mitochondria, endoplasmic reticulum and nuclear envelope: possible control points in apoptosis.J Mol Histol. 2004 Jan;35(1):11-9. doi: 10.1023/b:hijo.0000020900.86650.89. J Mol Histol. 2004. PMID: 15323345
-
CDIP1-BAP31 complex transduces apoptotic signals from endoplasmic reticulum to mitochondria under endoplasmic reticulum stress.Cell Rep. 2013 Oct 31;5(2):331-9. doi: 10.1016/j.celrep.2013.09.020. Epub 2013 Oct 17. Cell Rep. 2013. PMID: 24139803 Free PMC article.
-
The Multifunctional Sorting Protein PACS-2 Controls Mitophagosome Formation in Human Vascular Smooth Muscle Cells through Mitochondria-ER Contact Sites.Cells. 2019 Jun 25;8(6):638. doi: 10.3390/cells8060638. Cells. 2019. PMID: 31242668 Free PMC article.
-
Oxidative protein folding in the endoplasmic reticulum: tight links to the mitochondria-associated membrane (MAM).Biochim Biophys Acta. 2010 Aug;1798(8):1465-73. doi: 10.1016/j.bbamem.2010.04.009. Epub 2010 Apr 27. Biochim Biophys Acta. 2010. PMID: 20430008 Free PMC article. Review.
-
PACS-2: A key regulator of mitochondria-associated membranes (MAMs).Pharmacol Res. 2020 Oct;160:105080. doi: 10.1016/j.phrs.2020.105080. Epub 2020 Jul 13. Pharmacol Res. 2020. PMID: 32673704 Review.
Cited by
-
Superresolution imaging of viral protein trafficking.Med Microbiol Immunol. 2015 Jun;204(3):449-60. doi: 10.1007/s00430-015-0395-0. Epub 2015 Feb 28. Med Microbiol Immunol. 2015. PMID: 25724304 Free PMC article. Review.
-
Mitochondrial Contact Sites in Inflammation-Induced Cardiovascular Disease.Front Cell Dev Biol. 2020 Jul 30;8:692. doi: 10.3389/fcell.2020.00692. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32903766 Free PMC article. Review.
-
Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer's disease and related models.Proc Natl Acad Sci U S A. 2013 May 7;110(19):7916-21. doi: 10.1073/pnas.1300677110. Epub 2013 Apr 25. Proc Natl Acad Sci U S A. 2013. PMID: 23620518 Free PMC article.
-
Interaction with the effector dynamin-related protein 1 (Drp1) is an ancient function of Rab32 subfamily proteins.Cell Logist. 2014 Oct 2;4(4):e986399. doi: 10.4161/21592799.2014.986399. eCollection 2014 Oct-Dec. Cell Logist. 2014. PMID: 25767741 Free PMC article.
-
Using natural variation in Drosophila to discover previously unknown endoplasmic reticulum stress genes.Proc Natl Acad Sci U S A. 2013 May 28;110(22):9013-8. doi: 10.1073/pnas.1307125110. Epub 2013 May 10. Proc Natl Acad Sci U S A. 2013. PMID: 23667151 Free PMC article.
References
-
- Arvidson B, Seeds J, Webb M, Finlay L, Barklis E (2003) Analysis of the retrovirus capsid interdomain linker region. Virology 308: 166–177 - PubMed
-
- Berridge MJ (2002) The endoplasmic reticulum: a multifunctional signaling organelle. Cell Calcium 32: 235–249 - PubMed
-
- Blagoveshchenskaya AD, Thomas L, Feliciangeli SF, Hung CH, Thomas G (2002) HIV-1 Nef downregulates MHC-I by a PACS-1- and PI3K-regulated ARF6 endocytic pathway. Cell 111: 853–866 - PubMed
-
- Boatright KM, Salvesen GS (2003) Mechanisms of caspase activation. Curr Opin Cell Biol 15: 725–731 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

