Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells
- PMID: 15687132
- PMCID: PMC2754569
- DOI: 10.1161/01.CIR.0000153847.47301.80
Injectable self-assembling peptide nanofibers create intramyocardial microenvironments for endothelial cells
Abstract
Background: Promoting survival of transplanted cells or endogenous precursors is an important goal. We hypothesized that a novel approach to promote vascularization would be to create injectable microenvironments within the myocardium that recruit endothelial cells and promote their survival and organization.
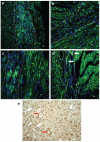
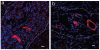
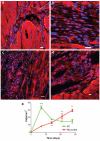
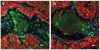
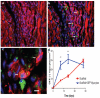
Methods and results: In this study we demonstrate that self-assembling peptides can be injected and that the resulting nanofiber microenvironments are readily detectable within the myocardium. Furthermore, the self-assembling peptide nanofiber microenvironments recruit progenitor cells that express endothelial markers, as determined by staining with isolectin and for the endothelial-specific protein platelet-endothelial cell adhesion molecule-1. Vascular smooth muscle cells are recruited to the microenvironment and appear to form functional vascular structures. After the endothelial cell population, cells that express alpha-sarcomeric actin and the transcription factor Nkx2.5 infiltrate the peptide microenvironment. When exogenous donor green fluorescent protein-positive neonatal cardiomyocytes were injected with the self-assembling peptides, transplanted cardiomyocytes in the peptide microenvironment survived and also augmented endogenous cell recruitment.
Conclusions: These experiments demonstrate that self-assembling peptides can create nanofiber microenvironments in the myocardium and that these microenvironments promote vascular cell recruitment. Because these peptide nanofibers may be modified in a variety of ways, this approach may enable injectable tissue regeneration strategies.
Figures



Similar articles
-
Transplantation of marrow-derived cardiac stem cells carried in designer self-assembling peptide nanofibers improves cardiac function after myocardial infarction.Biochem Biophys Res Commun. 2010 Aug 13;399(1):42-8. doi: 10.1016/j.bbrc.2010.07.031. Epub 2010 Jul 15. Biochem Biophys Res Commun. 2010. PMID: 20637726
-
Survival and maturation of human embryonic stem cell-derived cardiomyocytes in rat hearts.J Mol Cell Cardiol. 2007 Oct;43(4):504-16. doi: 10.1016/j.yjmcc.2007.07.001. Epub 2007 Jul 14. J Mol Cell Cardiol. 2007. PMID: 17707399 Free PMC article.
-
Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion.Circ Res. 2005 Jan 7;96(1):127-37. doi: 10.1161/01.RES.0000151843.79801.60. Epub 2004 Nov 29. Circ Res. 2005. PMID: 15569828
-
Human embryonic stem cell-derived vascular smooth muscle cells in therapeutic neovascularisation.J Mol Cell Cardiol. 2011 Nov;51(5):651-64. doi: 10.1016/j.yjmcc.2011.07.014. Epub 2011 Jul 24. J Mol Cell Cardiol. 2011. PMID: 21816157 Review.
-
Self-assembling peptide-based delivery of therapeutics for myocardial infarction.Adv Drug Deliv Rev. 2016 Jan 15;96:40-53. doi: 10.1016/j.addr.2015.04.023. Epub 2015 May 7. Adv Drug Deliv Rev. 2016. PMID: 25959427 Review.
Cited by
-
Self-assembling peptides-based nano-cargos for targeted chemotherapy and immunotherapy of tumors: recent developments, challenges, and future perspectives.Drug Deliv. 2022 Dec;29(1):1184-1200. doi: 10.1080/10717544.2022.2058647. Drug Deliv. 2022. PMID: 35403517 Free PMC article. Review.
-
Supramolecular Peptide Nanofiber Hydrogels for Bone Tissue Engineering: From Multihierarchical Fabrications to Comprehensive Applications.Adv Sci (Weinh). 2022 Apr;9(11):e2103820. doi: 10.1002/advs.202103820. Epub 2022 Feb 7. Adv Sci (Weinh). 2022. PMID: 35128831 Free PMC article. Review.
-
Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells.Proc Natl Acad Sci U S A. 2007 May 8;104(19):7791-6. doi: 10.1073/pnas.0701980104. Epub 2007 Apr 30. Proc Natl Acad Sci U S A. 2007. PMID: 17470802 Free PMC article.
-
Modification of hydrophilic and hydrophobic surfaces using an ionic-complementary peptide.PLoS One. 2007 Dec 19;2(12):e1325. doi: 10.1371/journal.pone.0001325. PLoS One. 2007. PMID: 18091996 Free PMC article.
-
Intra-myocardial biomaterial injection therapy in the treatment of heart failure: Materials, outcomes and challenges.Acta Biomater. 2011 Jan;7(1):1-15. doi: 10.1016/j.actbio.2010.06.039. Epub 2010 Jul 7. Acta Biomater. 2011. PMID: 20619368 Free PMC article. Review.
References
-
- Mann BK, West JL. Tissue engineering in the cardiovascular system: progress toward a tissue engineered heart. Anat Rec. 2001;263:367–371. - PubMed
-
- Li RK, Jia ZQ, Weisel RD, Mickle DA, Zhang J, Mohabeer MK, Rao V, Ivanov J. Cardiomyocyte transplantation improves heart function. Ann Thorac Surg. 1996;62:654–660. discussion 660–661. - PubMed
-
- Sakai T, Li RK, Weisel RD, Mickle DA, Kim EJ, Tomita S, Jia ZQ, Yau TM. Autologous heart cell transplantation improves cardiac function after myocardial injury. Ann Thorac Surg. 1999;68:2074–2080. discussion 2080–2081. - PubMed
-
- Papadaki M, Bursac N, Langer R, Merok J, Vunjak-Novakovic G, Freed LE. Tissue engineering of functional cardiac muscle: molecular, structural, and electrophysiological studies. Am J Physiol. 2001;280:H168–H178. - PubMed
-
- Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Neuhuber WL, Eschenhagen T. Tissue engineering of a differentiated cardiac muscle construct. Circ Res. 2002;90:223–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

