Adenovirus protein VII functions throughout early phase and interacts with cellular proteins SET and pp32
- PMID: 15681448
- PMCID: PMC546597
- DOI: 10.1128/JVI.79.4.2474-2483.2005
Adenovirus protein VII functions throughout early phase and interacts with cellular proteins SET and pp32
Abstract
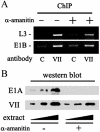
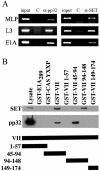
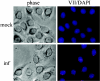
Adenovirus protein VII is the major component of the viral nucleoprotein core. It is a highly basic nonspecific DNA-binding protein that condenses viral DNA inside the capsid. We have investigated the fate and function of protein VII during infection. "Input" protein VII persisted in the nucleus throughout early phase and the beginning of DNA replication. Chromatin immunoprecipitation revealed that input protein VII remained associated with viral DNA during this period. Two cellular proteins, SET and pp32, also associated with viral DNA during early phase. They are components of two multiprotein complexes, the SET and INHAT complexes, implicated in chromatin-related activities. Protein VII associated with SET and pp32 in vitro and distinct domains of protein VII were responsible for binding to the two proteins. Interestingly, protein VII was found in novel nuclear dot structures as visualized by immunofluorescence. The dots likely represent individual infectious genomes in association with protein VII. They appeared within 30 min after infection and localized in the nucleus with a peak of intensity between 4 and 10 h postinfection. After this, their intensity decreased and they disappeared between 16 and 24 h postinfection. Interestingly, disappearance of the dots required ongoing RNA synthesis but not DNA synthesis. Taken together these data indicate that protein VII has an ongoing role during early phase and the beginning of DNA replication.
Figures
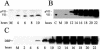




Similar articles
-
Transcription releases protein VII from adenovirus chromatin.Virology. 2007 Dec 20;369(2):411-22. doi: 10.1016/j.virol.2007.08.012. Epub 2007 Sep 20. Virology. 2007. PMID: 17888479
-
Adenovirus protein VII condenses DNA, represses transcription, and associates with transcriptional activator E1A.J Virol. 2004 Jun;78(12):6459-68. doi: 10.1128/JVI.78.12.6459-6468.2004. J Virol. 2004. PMID: 15163739 Free PMC article.
-
Default assembly of early adenovirus chromatin.Virology. 2007 Mar 1;359(1):116-25. doi: 10.1016/j.virol.2006.09.005. Epub 2006 Oct 10. Virology. 2007. PMID: 17034827
-
Epigenetics and the dynamics of chromatin during adenovirus infections.FEBS Lett. 2019 Dec;593(24):3551-3570. doi: 10.1002/1873-3468.13697. Epub 2019 Dec 15. FEBS Lett. 2019. PMID: 31769503 Free PMC article. Review.
-
Structure and function of nonhistone phosphoproteins.Biochem Cell Biol. 1988 May;66(5):349-66. doi: 10.1139/o88-043. Biochem Cell Biol. 1988. PMID: 3044396 Review.
Cited by
-
Altering the Ad5 packaging domain affects the maturation of the Ad particles.PLoS One. 2011;6(5):e19564. doi: 10.1371/journal.pone.0019564. Epub 2011 May 18. PLoS One. 2011. PMID: 21611162 Free PMC article.
-
Changes in adenoviral chromatin organization precede early gene activation upon infection.EMBO J. 2023 Oct 4;42(19):e114162. doi: 10.15252/embj.2023114162. Epub 2023 Aug 29. EMBO J. 2023. PMID: 37641864 Free PMC article.
-
A viral histone-like protein exploits antagonism between linker histones and HMGB proteins to obstruct the cell cycle.Curr Biol. 2021 Dec 6;31(23):5227-5237.e7. doi: 10.1016/j.cub.2021.09.050. Epub 2021 Oct 18. Curr Biol. 2021. PMID: 34666003 Free PMC article.
-
Human Adenovirus Infection Causes Cellular E3 Ubiquitin Ligase MKRN1 Degradation Involving the Viral Core Protein pVII.J Virol. 2018 Jan 17;92(3):e01154-17. doi: 10.1128/JVI.01154-17. Print 2018 Feb 1. J Virol. 2018. PMID: 29142133 Free PMC article.
-
Reduced infectivity of adenovirus type 5 particles and degradation of entering viral genomes associated with incomplete processing of the preterminal protein.J Virol. 2012 Dec;86(24):13554-65. doi: 10.1128/JVI.02337-12. Epub 2012 Oct 3. J Virol. 2012. PMID: 23035217 Free PMC article.
References
-
- Amin, M., A. Mirza, and J. Weber. 1977. Genetic analysis of adenovirus type 2. VII. Cleavage-modified affinity for DNA of internal virion proteins. Virology 80:83-97. - PubMed
-
- Beresford, P. J., D. Zhang, D. Y. Oh, Z. Fan, E. L. Greer, M. L. Russo, M. Jaju, and J. Lieberman. 2001. Granzyme A activates an endoplasmic reticulum-associated caspase-independent nuclease to induce single-stranded DNA nicks. J. Biol. Chem. 276:43285-43293. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

