Heterodimeric amino acid transporter glycoprotein domains determining functional subunit association
- PMID: 15679469
- PMCID: PMC1138950
- DOI: 10.1042/BJ20050021
Heterodimeric amino acid transporter glycoprotein domains determining functional subunit association
Abstract
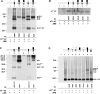
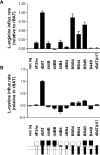
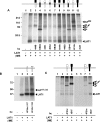
The heteromeric amino acid transporter glycoprotein subunits rBAT and 4F2hc (heavy chains) form, with different catalytic subunits (light chains), functional heterodimers that are covalently stabilized by a disulphide bridge. Whereas rBAT associates with b(0,+)AT to form the cystine and cationic amino acid transporter defective in cystinuria, 4F2hc associates with other homologous light chains, for instance with LAT1 to form a system L neutral amino acid transporter. To identify within the heavy chains the domain(s) involved in recognition of and functional interaction with partner light chains, chimaeric and truncated forms of rBAT and 4F2hc were co-expressed in Xenopus laevis oocytes with b(0,+)AT or LAT1. Heavy chain-light chain association was analysed by co-immunoprecipitation, and transport function was tested by tracer uptake experiments. The results indicate that the cytoplasmic tail and transmembrane domain of rBAT together play a dominant role in selective functional interaction with b(0,+)AT, whereas the extracellular domain of rBAT appears to facilitate specifically L-cystine uptake. For 4F2hc, functional interaction with LAT1 was mediated by the N-terminal part, comprising cytoplasmic tail, transmembrane segment and neck, even in the absence of the extracellular domain. Alternatively, functional association with LAT1 was also supported by the extracellular part of 4F2hc comprising neck and glycosidase-like domain linked to the complementary part of rBAT. In conclusion, the cytoplasmic tail and the transmembrane segment together play a determinant role for the functional interaction of rBAT with b(0,+)AT, whereas either cytoplasmic or extracellular glycosidase-like domains are dispensable for the functional interaction of 4F2hc with LAT1.
Figures
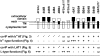

Similar articles
-
Expression of heteromeric amino acid transporters along the murine intestine.J Physiol. 2004 Jul 15;558(Pt 2):597-610. doi: 10.1113/jphysiol.2004.065037. Epub 2004 May 21. J Physiol. 2004. PMID: 15155792 Free PMC article.
-
Association of 4F2hc with light chains LAT1, LAT2 or y+LAT2 requires different domains.Biochem J. 2001 May 1;355(Pt 3):725-31. doi: 10.1042/bj3550725. Biochem J. 2001. PMID: 11311135 Free PMC article.
-
Amino acid transport of y+L-type by heterodimers of 4F2hc/CD98 and members of the glycoprotein-associated amino acid transporter family.EMBO J. 1999 Jan 4;18(1):49-57. doi: 10.1093/emboj/18.1.49. EMBO J. 1999. PMID: 9878049 Free PMC article.
-
Structure-function relationships of heterodimeric amino acid transporters.Cell Biochem Biophys. 2002;36(2-3):155-68. doi: 10.1385/CBB:36:2-3:155. Cell Biochem Biophys. 2002. PMID: 12139401 Review.
-
Function and structure of heterodimeric amino acid transporters.Am J Physiol Cell Physiol. 2001 Oct;281(4):C1077-93. doi: 10.1152/ajpcell.2001.281.4.C1077. Am J Physiol Cell Physiol. 2001. PMID: 11546643 Review.
Cited by
-
Pathophysiology and treatment of cystinuria.Nat Rev Nephrol. 2010 Jul;6(7):424-34. doi: 10.1038/nrneph.2010.69. Epub 2010 Jun 1. Nat Rev Nephrol. 2010. PMID: 20517292 Review.
-
Transport of Biologically Active Ultrashort Peptides Using POT and LAT Carriers.Int J Mol Sci. 2022 Jul 13;23(14):7733. doi: 10.3390/ijms23147733. Int J Mol Sci. 2022. PMID: 35887081 Free PMC article. Review.
-
Differential axial localization along the mouse brain vascular tree of luminal sodium-dependent glutamine transporters Snat1 and Snat3.J Cereb Blood Flow Metab. 2011 Jul;31(7):1637-47. doi: 10.1038/jcbfm.2011.21. Epub 2011 Mar 2. J Cereb Blood Flow Metab. 2011. PMID: 21364602 Free PMC article.
-
The zebrafish cationic amino acid transporter/glycoprotein-associated family: sequence and spatiotemporal distribution during development of the transport system b0,+ (slc3a1/slc7a9).Fish Physiol Biochem. 2021 Oct;47(5):1507-1525. doi: 10.1007/s10695-021-00984-z. Epub 2021 Aug 2. Fish Physiol Biochem. 2021. PMID: 34338990 Free PMC article.
-
SLC7A9 cDNA cloning and mutational analysis of SLC3A1 and SLC7A9 in canine cystinuria.Mamm Genome. 2006 Jul;17(7):769-76. doi: 10.1007/s00335-005-0146-4. Epub 2006 Jul 14. Mamm Genome. 2006. PMID: 16845473
References
-
- Wagner C. A., Lang F., Broer S. Function and structure of heterodimeric amino acid transporters. Am. J. Physiol. Cell Physiol. 2001;281:C1077–C1093. - PubMed
-
- Chillaron J., Roca R., Valencia A., Zorzano A., Palacin M. Heteromeric amino acid transporters: biochemistry, genetics, and physiology. Am. J. Physiol. Renal Physiol. 2001;281:F995–F1018. - PubMed
-
- Deves R., Boyd C. A. Surface antigen CD98(4F2): not a single membrane protein, but a family of proteins with multiple functions. J. Membr. Biol. 2000;173:165–177. - PubMed
-
- Verrey F., Jack D. L., Paulsen I. T., Saier M. H., Pfeiffer R. New glycoprotein-associated amino acid transporters. J. Membr. Biol. 1999;172:181–192. - PubMed
-
- Verrey F., Closs E. I., Wagner C. A., Palacin M., Endou H., Kanai Y. CATs and HATs: the SLC7 family of amino acid transporters. Pflugers Arch. Eur. J. Physiol. 2004;447:532–542. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

