Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells
- PMID: 15667565
- PMCID: PMC1782075
- DOI: 10.1111/j.1365-2567.2004.02076.x
Human monocyte isolation methods influence cytokine production from in vitro generated dendritic cells
Abstract
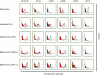
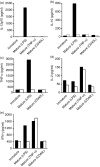
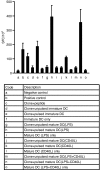
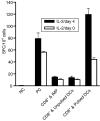
There is growing interest in the in vitro generation of dendritic cells (DC) from peripheral blood monocytes, but the effect of the method chosen to isolate CD14+ monocytes for subsequent DC generation is poorly documented. The method used to isolate monocytes may have an impact on the subsequent function of DC by affecting their ability to express costimulatory molecules (CD80/86), maturation marker (CD83) and/or to produce important immunomodulatory cytokines. In this study, we show that the positive selection of monocytes by anti-CD14-coated microbeads inhibits the lipopolysaccharide (LPS)-induced production of interleukin (IL)-12, IL-10 and tumour necrosis factor-alpha (TNF-alpha) from human DC. However, when DC were grown from monocytes isolated by plastic adherence, LPS induced the production of much higher levels of these cytokines. DC derived from adherence-isolated monocytes induced the development of potent cytotoxic T lymphocytes of the Tc1 subset specific for influenza matrix protein, as confirmed by interferon-gamma (IFN-gamma) enzyme-linked immunosorbent spot-forming cell assay (ELISPOT), cytotoxicity assay, major histocompatibility complex (MHC)-peptide tetrameric complexes and T helper 1/T helper 2 (Th1/Th2) cytokine production assays.
Figures


Similar articles
-
Effects of monomethylfumarate on dendritic cell differentiation.Br J Dermatol. 2006 Feb;154(2):211-7. doi: 10.1111/j.1365-2133.2005.07002.x. Br J Dermatol. 2006. PMID: 16433787
-
Role of the cytokine environment and cytokine receptor expression on the generation of functionally distinct dendritic cells from human monocytes.Eur J Immunol. 2008 Mar;38(3):750-62. doi: 10.1002/eji.200737395. Eur J Immunol. 2008. PMID: 18236400
-
Cytokine profiles of canine monocyte-derived dendritic cells as a function of lipopolysaccharide- or tumor necrosis factor-alpha-induced maturation.Vet Immunol Immunopathol. 2007 Aug 15;118(3-4):186-98. doi: 10.1016/j.vetimm.2007.05.010. Epub 2007 Jun 2. Vet Immunol Immunopathol. 2007. PMID: 17617471
-
Flow cytometric analysis of cytokine production by normal human peripheral blood dendritic cells and monocytes: comparative analysis of different stimuli, secretion-blocking agents and incubation periods.Cytometry. 2001 Feb 15;46(1):33-40. Cytometry. 2001. PMID: 11241505
-
CD40 engagement strongly induces CD25 expression on porcine dendritic cells and polarizes the T cell immune response toward Th1.Mol Immunol. 2009 Jan;46(3):437-47. doi: 10.1016/j.molimm.2008.10.014. Epub 2008 Nov 25. Mol Immunol. 2009. PMID: 19036453
Cited by
-
Bacillus anthracis' lethal toxin induces broad transcriptional responses in human peripheral monocytes.BMC Immunol. 2012 Jul 2;13:33. doi: 10.1186/1471-2172-13-33. BMC Immunol. 2012. PMID: 22747600 Free PMC article.
-
High-throughput measurement of single-cell growth rates using serial microfluidic mass sensor arrays.Nat Biotechnol. 2016 Oct;34(10):1052-1059. doi: 10.1038/nbt.3666. Epub 2016 Sep 5. Nat Biotechnol. 2016. PMID: 27598230 Free PMC article.
-
Dendritic cell immunizations alone or combined with low doses of interleukin-2 induce specific immune responses in melanoma patients.Clin Exp Immunol. 2005 Dec;142(3):555-68. doi: 10.1111/j.1365-2249.2005.02948.x. Clin Exp Immunol. 2005. PMID: 16297169 Free PMC article. Clinical Trial.
-
Comparison between magnetic activated cell sorted monocytes and monocyte adherence techniques for in vitro generation of immature dendritic cells: an Egyptian trial.Cent Eur J Immunol. 2015;40(1):18-24. doi: 10.5114/ceji.2015.50828. Epub 2015 Apr 22. Cent Eur J Immunol. 2015. PMID: 26155179 Free PMC article.
-
The generation and application of antigen-specific T cell therapies for cancer and viral-associated disease.Mol Ther. 2022 Jun 1;30(6):2130-2152. doi: 10.1016/j.ymthe.2022.02.002. Epub 2022 Feb 9. Mol Ther. 2022. PMID: 35149193 Free PMC article. Review.
References
-
- Lanzavecchia A, Sallusto F. The instructive role of dendritic cells on T cell responses: lineages, plasticity and kinetics. Curr Opin Immunol. 2001;13:291–8. 10.1016/S0952-7915(00)00218-1. - DOI - PubMed
-
- Steinman RM, Gutchinov B, Witmer MD, Nussenzweig MC. Dendritic cells are the principal stimulators of the primary mixed leukocyte reaction in mice. J Exp Med. 1983;157:613–27. 10.1084/jem.157.2.613. - DOI - PMC - PubMed
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. - PubMed
-
- Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. 10.1146/annurev.immunol.21.120601.141040. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

