A cascade of cytokines mediates mechanical inflammatory hypernociception in mice
- PMID: 15665080
- PMCID: PMC547882
- DOI: 10.1073/pnas.0409225102
A cascade of cytokines mediates mechanical inflammatory hypernociception in mice
Abstract
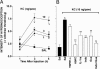
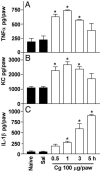
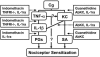
The hypernociceptive effects of cytokines [TNF-alpha, keratinocyte-derived chemokine (KC), and IL-1beta] and their participation in carrageenan (Cg)-induced inflammatory hypernociception in mice were investigated. Nociceptor sensitization (hypernociception) was quantified with an electronic version of the von Frey filament test in WT and TNF receptor type 1 knockout mice (TNF-R1-/-). TNF-alpha-induced hypernociception was abolished in TNF-R1-/- mice, partially inhibited by pretreatment with IL-1 receptor antagonist (IL-1ra) or indomethacin and unaffected by Ab against KC (AbKC) or guanethidine. IL-1ra and indomethacin pretreatment strongly inhibited the hypernociception induced by IL-1beta, which was not altered by AbKC or guanethidine or by knocking out TNF-R1. KC-induced hypernociception was abolished by AbKC, inhibited by pretreatment with indomethacin plus guanethidine, and partially inhibited by IL-1ra, indomethacin, or guanethidine. In contrast, KC-induced hypernociception was not altered by knocking out TNF-R1. Cg-induced hypernociception was abolished by administration of indomethacin plus guanethidine, diminished in TNF-R1-/- mice, and partially inhibited in WT mice pretreated with AbKC, IL-1ra, indomethacin, or guanethidine. TNF-alpha, KC, and IL-1beta concentrations were elevated in the skin of Cg-injected paws. The TNF-alpha and KC concentrations rose concomitantly and peaked before that of IL-1beta. In mice, the cytokine cascade begins with the release of TNF-alpha (acting on TNF-R1 receptor) and KC, which stimulate the release of IL-1beta. As in rats, the final mediators of this cascade were prostaglandins released by IL-1beta and sympathetic amines released by KC. These results extend to mice the concept that the release of primary mediators responsible for hypernociception is preceded by a cascade of cytokines.
Figures

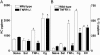


Similar articles
-
TNF-alpha and IL-1beta mediate inflammatory hypernociception in mice triggered by B1 but not B2 kinin receptor.Eur J Pharmacol. 2007 Nov 14;573(1-3):221-9. doi: 10.1016/j.ejphar.2007.07.007. Epub 2007 Jul 13. Eur J Pharmacol. 2007. PMID: 17669394
-
Role of cytokines in mediating mechanical hypernociception in a model of delayed-type hypersensitivity in mice.Eur J Pain. 2008 Nov;12(8):1059-68. doi: 10.1016/j.ejpain.2008.02.003. Epub 2008 Mar 26. Eur J Pain. 2008. PMID: 18372199
-
IL-17 mediates articular hypernociception in antigen-induced arthritis in mice.Pain. 2010 Feb;148(2):247-256. doi: 10.1016/j.pain.2009.11.006. Epub 2009 Dec 6. Pain. 2010. PMID: 19969421
-
Crucial role of neutrophils in the development of mechanical inflammatory hypernociception.J Leukoc Biol. 2008 Apr;83(4):824-32. doi: 10.1189/jlb.0907654. Epub 2008 Jan 18. J Leukoc Biol. 2008. PMID: 18203872
-
Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development?Pharmacol Ther. 2006 Oct;112(1):116-38. doi: 10.1016/j.pharmthera.2006.04.001. Epub 2006 May 30. Pharmacol Ther. 2006. PMID: 16730375 Review.
Cited by
-
Bioactive Fraction of Geopropolis from Melipona scutellaris Decreases Neutrophils Migration in the Inflammatory Process: Involvement of Nitric Oxide Pathway.Evid Based Complement Alternat Med. 2013;2013:907041. doi: 10.1155/2013/907041. Epub 2013 Apr 30. Evid Based Complement Alternat Med. 2013. PMID: 23737853 Free PMC article.
-
A study of the mechanisms underlying the anti-inflammatory effect of ellagic acid in carrageenan-induced paw edema in rats.Indian J Pharmacol. 2015 May-Jun;47(3):292-8. doi: 10.4103/0253-7613.157127. Indian J Pharmacol. 2015. PMID: 26069367 Free PMC article.
-
Nociceptor Sensory Neuron-Immune Interactions in Pain and Inflammation.Trends Immunol. 2017 Jan;38(1):5-19. doi: 10.1016/j.it.2016.10.001. Epub 2016 Oct 25. Trends Immunol. 2017. PMID: 27793571 Free PMC article. Review.
-
Drosophila cytokine GBP2 exerts immune responses and regulates GBP1 expression through GPCR receptor Mthl10.Insect Biochem Mol Biol. 2024 Apr;167:104086. doi: 10.1016/j.ibmb.2024.104086. Epub 2024 Jan 29. Insect Biochem Mol Biol. 2024. PMID: 38295885
-
The chemokine Bv8/prokineticin 2 is up-regulated in inflammatory granulocytes and modulates inflammatory pain.Proc Natl Acad Sci U S A. 2009 Aug 25;106(34):14646-51. doi: 10.1073/pnas.0903720106. Epub 2009 Aug 10. Proc Natl Acad Sci U S A. 2009. PMID: 19667192 Free PMC article.
References
-
- Blackwell, T. S. & Christman, J. W. (1996) Br. J. Anaesth. 77, 110–117. - PubMed
-
- Dinarello, C. A. (2000) Chest 118, 503–508. - PubMed
-
- Hopkins, S. J. (2003) Leg. Med. (Tokyo) 5, S45–S57. - PubMed
-
- Cunha, F. Q. & Ferreira, S. H. (2003) Adv. Exp. Med. Biol. 521, 22–39. - PubMed
-
- Conti, B., Tabarean, I., Andrei, C. & Bartfai, T. (2004) Front. Biosci. 9, 1433–1449. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

