Eukaryotic translation initiation factor 4E activity is modulated by HOXA9 at multiple levels
- PMID: 15657436
- PMCID: PMC544005
- DOI: 10.1128/MCB.25.3.1100-1112.2005
Eukaryotic translation initiation factor 4E activity is modulated by HOXA9 at multiple levels
Abstract
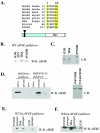
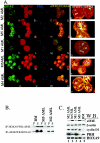
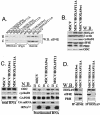
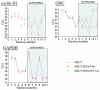
The eukaryotic translation initiation factor 4E (eIF4E) alters gene expression on multiple levels. In the cytoplasm, eIF4E acts in the rate-limiting step of translation initiation. In the nucleus, eIF4E facilitates nuclear export of a subset of mRNAs. Both of these functions contribute to eIF4E's ability to oncogenically transform cells. We report here that the homeodomain protein, HOXA9, is a positive regulator of eIF4E. HOXA9 stimulates eIF4E-dependent export of cyclin D1 and ornithine decarboxylase (ODC) mRNAs in the nucleus, as well as increases the translation efficiency of ODC mRNA in the cytoplasm. These activities depend on direct interactions of HOXA9 with eIF4E and are independent of the role of HOXA9 in transcription. At the biochemical level, HOXA9 mediates these effects by competing with factors that repress eIF4E function, in particular the proline-rich homeodomain PRH/Hex. This competitive mechanism of eIF4E regulation is disrupted in a subset of leukemias, where HOXA9 displaces PRH from eIF4E, thereby contributing to eIF4E's dysregulation. In regard to these results and our previous finding that approximately 200 homeodomain proteins contain eIF4E binding sites, we propose that homeodomain modulation of eIF4E activity is a novel means through which this family of proteins implements their effects on growth and development.
Figures

Similar articles
-
The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth.EMBO J. 2003 Feb 3;22(3):689-703. doi: 10.1093/emboj/cdg069. EMBO J. 2003. PMID: 12554669 Free PMC article.
-
Aberrant eukaryotic translation initiation factor 4E-dependent mRNA transport impedes hematopoietic differentiation and contributes to leukemogenesis.Mol Cell Biol. 2003 Dec;23(24):8992-9002. doi: 10.1128/MCB.23.24.8992-9002.2003. Mol Cell Biol. 2003. PMID: 14645512 Free PMC article.
-
The emerging roles of translation factor eIF4E in the nucleus.Differentiation. 2002 Mar;70(1):10-22. doi: 10.1046/j.1432-0436.2002.700102.x. Differentiation. 2002. PMID: 11963652 Review.
-
eIF4E promotes nuclear export of cyclin D1 mRNAs via an element in the 3'UTR.J Cell Biol. 2005 Apr 25;169(2):245-56. doi: 10.1083/jcb.200501019. Epub 2005 Apr 18. J Cell Biol. 2005. PMID: 15837800 Free PMC article.
-
The eukaryotic translation initiation factor eIF4E in the nucleus: taking the road less traveled.Immunol Rev. 2015 Jan;263(1):210-23. doi: 10.1111/imr.12240. Immunol Rev. 2015. PMID: 25510279 Free PMC article. Review.
Cited by
-
Dual functions of the homeoprotein DLX4 in modulating responsiveness of tumor cells to topoisomerase II-targeting drugs.Cancer Res. 2013 Jan 15;73(2):1000-10. doi: 10.1158/0008-5472.CAN-12-3538. Epub 2012 Dec 7. Cancer Res. 2013. PMID: 23222298 Free PMC article.
-
Tracking a refined eIF4E-binding motif reveals Angel1 as a new partner of eIF4E.Nucleic Acids Res. 2013 Sep;41(16):7783-92. doi: 10.1093/nar/gkt569. Epub 2013 Jun 27. Nucleic Acids Res. 2013. PMID: 23814182 Free PMC article.
-
Structural characterization of the Z RING-eIF4E complex reveals a distinct mode of control for eIF4E.Proc Natl Acad Sci U S A. 2010 Mar 23;107(12):5441-6. doi: 10.1073/pnas.0909877107. Epub 2010 Mar 8. Proc Natl Acad Sci U S A. 2010. PMID: 20212144 Free PMC article.
-
A Case of Identity: HOX Genes in Normal and Cancer Stem Cells.Cancers (Basel). 2019 Apr 10;11(4):512. doi: 10.3390/cancers11040512. Cancers (Basel). 2019. PMID: 30974862 Free PMC article. Review.
-
eIF4E orchestrates mRNA processing, RNA export and translation to modify specific protein production.Nucleus. 2024 Dec;15(1):2360196. doi: 10.1080/19491034.2024.2360196. Epub 2024 Jun 16. Nucleus. 2024. PMID: 38880976 Free PMC article. Review.
References
-
- Calvo, K. R., D. B. Sykes, M. Pasillas, and M. P. Kamps. 2000. Hoxa9 immortalizes a granulocyte-macrophage colony-stimulating factor-dependent promyelocyte capable of biphenotypic differentiation to neutrophils or macrophages, independent of enforced Meis expression. Mol. Cell. Biol. 20:3274-3285. - PMC - PubMed
-
- Clemens, M. J., and U. A. Bommer. 1999. Translational control: the cancer connection. Int. J. Biochem. Cell Biol. 31:1-23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
