A novel mechanism of plasmin-induced mitogenesis in fibroblasts
- PMID: 15634280
- PMCID: PMC2829869
- DOI: 10.1111/j.1538-7836.2004.01054.x
A novel mechanism of plasmin-induced mitogenesis in fibroblasts
Abstract
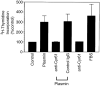
The plasminogen activator/plasmin system is believed to play an important role in diverse pathophysiological processes, including wound healing, vascular remodeling and pulmonary fibrosis. Our recent studies show that plasmin upregulates the expression of Cyr61, a growth factor-like gene that has been implicated in cell proliferation and migration. In the present study, we investigated whether plasmin promotes fibroblast proliferation and, if so, determine the role of Cyr61 in the plasmin-induced response. Human lung fibroblasts were exposed to varying concentrations of plasmin and DNA synthesis was monitored by measuring the incorporation of 3H-thymidine into DNA. Plasmin increased DNA synthesis of fibroblasts in a dose-dependent manner. Protease-activated receptor-1 (PAR-1)-specific antibodies, but not PAR-2-specific antibodies, reduced the plasmin-induced DNA synthesis. Consistent with this, plasmin had no substantial effect on the DNA synthesis in PAR-1-deficient mouse fibroblasts. Plasmin activated both p38 and p44/42 MAPKs and specific inhibitors of these pathways inhibited the plasmin-induced DNA synthesis. Plasmin-induced increase in the DNA synthesis was completely abrogated by anti-Cyr61 antibodies. Interestingly, thrombin, which is a potent inducer of Cyr61, had only a minimal effect on fibroblast proliferation. Additional experiments suggested that plasmin cleaved cell/extracellular matrix-associated Cyr61 and the conditioned media from plasmin-treated cells could support the cell proliferation. Overall, these data suggest that plasmin promotes fibroblast proliferation by a novel pathway, involving two independent steps. In the first step, plasmin induces Cyr61 expression via activation of PAR-1, and in the second step, plasmin releases Cyr61 deposited in the extracellular matrix, thus making it accessible to act on cells.
Figures
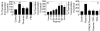
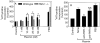
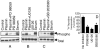

Similar articles
-
Plasmin induces Cyr61 gene expression in fibroblasts via protease-activated receptor-1 and p44/42 mitogen-activated protein kinase-dependent signaling pathway.Arterioscler Thromb Vasc Biol. 2002 Sep 1;22(9):1421-6. doi: 10.1161/01.atv.0000030200.59331.3f. Arterioscler Thromb Vasc Biol. 2002. PMID: 12231560
-
The angiogenic factor Cyr61 activates a genetic program for wound healing in human skin fibroblasts.J Biol Chem. 2001 Dec 14;276(50):47329-37. doi: 10.1074/jbc.M107666200. Epub 2001 Oct 2. J Biol Chem. 2001. PMID: 11584015
-
The angiogenic factors Cyr61 and connective tissue growth factor induce adhesive signaling in primary human skin fibroblasts.J Biol Chem. 2001 Mar 30;276(13):10443-52. doi: 10.1074/jbc.M008087200. Epub 2000 Dec 18. J Biol Chem. 2001. PMID: 11120741
-
Thrombin alters the synthesis and processing of CYR61/CCN1 in human corneal stromal fibroblasts and myofibroblasts through multiple distinct mechanisms.Mol Vis. 2020 Jul 29;26:540-562. eCollection 2020. Mol Vis. 2020. PMID: 32818017 Free PMC article.
-
CYR61 stimulates human skin fibroblast migration through Integrin alpha vbeta 5 and enhances mitogenesis through integrin alpha vbeta 3, independent of its carboxyl-terminal domain.J Biol Chem. 2001 Jun 15;276(24):21943-50. doi: 10.1074/jbc.M100978200. Epub 2001 Apr 3. J Biol Chem. 2001. PMID: 11287419
Cited by
-
The antifibrotic effects of plasminogen activation occur via prostaglandin E2 synthesis in humans and mice.J Clin Invest. 2010 Jun;120(6):1950-60. doi: 10.1172/JCI38369. Epub 2010 May 24. J Clin Invest. 2010. PMID: 20501949 Free PMC article.
-
Cell surface remodeling by plasmin: a new function for an old enzyme.J Biomed Biotechnol. 2012;2012:564259. doi: 10.1155/2012/564259. Epub 2012 Oct 14. J Biomed Biotechnol. 2012. PMID: 23097597 Free PMC article. Review.
-
Tissue factor-factor VIIa signaling.Arterioscler Thromb Vasc Biol. 2005 Jan;25(1):47-56. doi: 10.1161/01.ATV.0000151624.45775.13. Epub 2004 Nov 29. Arterioscler Thromb Vasc Biol. 2005. PMID: 15569823 Free PMC article. Review.
-
Critical Role of Cysteine-Rich Protein 61 in Mediating the Activation of Renal Fibroblasts.Front Physiol. 2019 May 3;10:464. doi: 10.3389/fphys.2019.00464. eCollection 2019. Front Physiol. 2019. PMID: 31130867 Free PMC article.
-
Plasminogen activation induced pericellular fibronectin proteolysis promotes fibroblast apoptosis.Am J Respir Cell Mol Biol. 2008 Jan;38(1):78-87. doi: 10.1165/rcmb.2007-0174OC. Epub 2007 Jul 26. Am J Respir Cell Mol Biol. 2008. PMID: 17656680 Free PMC article.
References
-
- Carmeliet P, Collen D. Development and disease in proteinase-deficient mice: role of the plasminogen, matrix metalloproteinase and coagulation system. Thromb Res. 1998;91:255–85. - PubMed
-
- Pepper MS. Role of the matrix metalloproteinases and plasminogen activator–plasmin system in angiogenesis. Arterioscler Thromb Vasc Biol. 2001;21:1104–17. - PubMed
-
- Lijnen HR. Plasmin and matrix metalloproteinases. Thromb Haemost. 2001;86:324–33. - PubMed
-
- Ploplis VA, Castellino FJ. Gene targeting of components of the fibrinolytic system. Thromb Haemost. 2002;87:22–31. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

