Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice
- PMID: 15619330
- PMCID: PMC544401
- DOI: 10.1186/1472-6750-4-33
Dynamic in vivo imaging and cell tracking using a histone fluorescent protein fusion in mice
Abstract
Background: Advances in optical imaging modalities and the continued evolution of genetically-encoded fluorescent proteins are coming together to facilitate the study of cell behavior at high resolution in living organisms. As a result, imaging using autofluorescent protein reporters is gaining popularity in mouse transgenic and targeted mutagenesis applications.
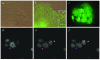
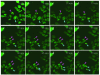
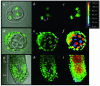
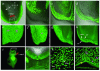
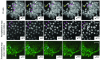
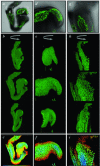
Results: We have used embryonic stem cell-mediated transgenesis to label cells at sub-cellular resolution in vivo, and to evaluate fusion of a human histone protein to green fluorescent protein for ubiquitous fluorescent labeling of nucleosomes in mice. To this end we have generated embryonic stem cells and a corresponding strain of mice that is viable and fertile and exhibits widespread chromatin-localized reporter expression. High levels of transgene expression are maintained in a constitutive manner. Viability and fertility of homozygous transgenic animals demonstrates that this reporter is developmentally neutral and does not interfere with mitosis or meiosis.
Conclusions: Using various optical imaging modalities including wide-field, spinning disc confocal, and laser scanning confocal and multiphoton excitation microscopy, we can identify cells in various stages of the cell cycle. We can identify cells in interphase, cells undergoing mitosis or cell death. We demonstrate that this histone fusion reporter allows the direct visualization of active chromatin in situ. Since this reporter segments three-dimensional space, it permits the visualization of individual cells within a population, and so facilitates tracking cell position over time. It is therefore attractive for use in multidimensional studies of in vivo cell behavior and cell fate.
Figures

Similar articles
-
Genetic and spectrally distinct in vivo imaging: embryonic stem cells and mice with widespread expression of a monomeric red fluorescent protein.BMC Biotechnol. 2005 Jul 4;5:20. doi: 10.1186/1472-6750-5-20. BMC Biotechnol. 2005. PMID: 15996270 Free PMC article.
-
Using a histone yellow fluorescent protein fusion for tagging and tracking endothelial cells in ES cells and mice.Genesis. 2005 Jul;42(3):162-71. doi: 10.1002/gene.20139. Genesis. 2005. PMID: 15986455 Free PMC article.
-
Dual transgene strategy for live visualization of chromatin and plasma membrane dynamics in murine embryonic stem cells and embryonic tissues.Genesis. 2009 May;47(5):330-6. doi: 10.1002/dvg.20500. Genesis. 2009. PMID: 19358158 Free PMC article.
-
Rational design of genetically encoded reporter genes for optical imaging of apoptosis.Apoptosis. 2020 Aug;25(7-8):459-473. doi: 10.1007/s10495-020-01621-5. Apoptosis. 2020. PMID: 32623548 Review.
-
Technicolour transgenics: imaging tools for functional genomics in the mouse.Nat Rev Genet. 2003 Aug;4(8):613-25. doi: 10.1038/nrg1126. Nat Rev Genet. 2003. PMID: 12897773 Review.
Cited by
-
Live Tissue Imaging Sheds Light on Cell Level Events During Ectodermal Organ Development.Front Physiol. 2020 Jul 16;11:818. doi: 10.3389/fphys.2020.00818. eCollection 2020. Front Physiol. 2020. PMID: 32765297 Free PMC article. Review.
-
Multiple requirements of PLK1 during mouse oocyte maturation.PLoS One. 2015 Feb 6;10(2):e0116783. doi: 10.1371/journal.pone.0116783. eCollection 2015. PLoS One. 2015. PMID: 25658810 Free PMC article.
-
Growth-factor-mediated coupling between lineage size and cell fate choice underlies robustness of mammalian development.Elife. 2020 Jul 28;9:e56079. doi: 10.7554/eLife.56079. Elife. 2020. PMID: 32720894 Free PMC article.
-
R26R-GR: a Cre-activable dual fluorescent protein reporter mouse.PLoS One. 2012;7(9):e46171. doi: 10.1371/journal.pone.0046171. Epub 2012 Sep 25. PLoS One. 2012. PMID: 23049968 Free PMC article.
-
An ex vivo system to study cellular dynamics underlying mouse peri-implantation development.Dev Cell. 2022 Feb 7;57(3):373-386.e9. doi: 10.1016/j.devcel.2021.12.023. Epub 2022 Jan 20. Dev Cell. 2022. PMID: 35063082 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

