Natural history of a recurrent feline coronavirus infection and the role of cellular immunity in survival and disease
- PMID: 15613332
- PMCID: PMC538555
- DOI: 10.1128/JVI.79.2.1036-1044.2005
Natural history of a recurrent feline coronavirus infection and the role of cellular immunity in survival and disease
Abstract
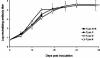
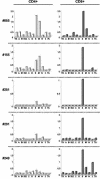
We describe the natural history, viral dynamics, and immunobiology of feline infectious peritonitis (FIP), a highly lethal coronavirus infection. A severe recurrent infection developed, typified by viral persistence and acute lymphopenia, with waves of enhanced viral replication coinciding with fever, weight loss, and depletion of CD4+ and CD8+ T cells. Our combined observations suggest a model for FIP pathogenesis in which virus-induced T-cell depletion and the antiviral T-cell response are opposing forces and in which the efficacy of early T-cell responses critically determines the outcome of the infection. Rising amounts of viral RNA in the blood, consistently seen in animals with end-stage FIP, indicate that progression to fatal disease is the direct consequence of a loss of immune control, resulting in unchecked viral replication. The pathogenic phenomena described here likely bear relevance to other severe coronavirus infections, in particular severe acute respiratory syndrome, for which multiphasic disease progression and acute T-cell lymphopenia have also been reported. Experimental FIP presents a relevant, safe, and well-defined model to study coronavirus-mediated immunosuppression and should provide an attractive and convenient system for in vivo testing of anticoronaviral drugs.
Figures
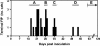



Similar articles
-
Characterization of antiviral T cell responses during primary and secondary challenge of laboratory cats with feline infectious peritonitis virus (FIPV).BMC Vet Res. 2019 May 22;15(1):165. doi: 10.1186/s12917-019-1909-6. BMC Vet Res. 2019. PMID: 31118053 Free PMC article.
-
Reversal of the Progression of Fatal Coronavirus Infection in Cats by a Broad-Spectrum Coronavirus Protease Inhibitor.PLoS Pathog. 2016 Mar 30;12(3):e1005531. doi: 10.1371/journal.ppat.1005531. eCollection 2016 Mar. PLoS Pathog. 2016. PMID: 27027316 Free PMC article.
-
Establishment of a Virulent Full-Length cDNA Clone for Type I Feline Coronavirus Strain C3663.J Virol. 2019 Oct 15;93(21):e01208-19. doi: 10.1128/JVI.01208-19. Print 2019 Nov 1. J Virol. 2019. PMID: 31375588 Free PMC article.
-
Feline infectious peritonitis: still an enigma?Vet Pathol. 2014 Mar;51(2):505-26. doi: 10.1177/0300985814522077. Vet Pathol. 2014. PMID: 24569616 Review.
-
An update on feline infectious peritonitis: virology and immunopathogenesis.Vet J. 2014 Aug;201(2):123-32. doi: 10.1016/j.tvjl.2014.04.017. Epub 2014 May 2. Vet J. 2014. PMID: 24837550 Free PMC article. Review.
Cited by
-
Comparison of Antiviral Immune Responses in Healthy Cats Induced by Two Immune Therapeutics.Pathogens. 2024 Jul 22;13(7):602. doi: 10.3390/pathogens13070602. Pathogens. 2024. PMID: 39057828 Free PMC article.
-
Feline Infectious Peritonitis: European Advisory Board on Cat Diseases Guidelines.Viruses. 2023 Aug 31;15(9):1847. doi: 10.3390/v15091847. Viruses. 2023. PMID: 37766254 Free PMC article. Review.
-
Retrospective study and outcome of 307 cats with feline infectious peritonitis treated with legally sourced veterinary compounded preparations of remdesivir and GS-441524 (2020-2022).J Feline Med Surg. 2023 Sep;25(9):1098612X231194460. doi: 10.1177/1098612X231194460. J Feline Med Surg. 2023. PMID: 37732386 Free PMC article.
-
Towards Understanding Long COVID: SARS-CoV-2 Strikes the Host Cell Nucleus.Pathogens. 2023 Jun 6;12(6):806. doi: 10.3390/pathogens12060806. Pathogens. 2023. PMID: 37375496 Free PMC article. Review.
-
Effect of GS-441524 in combination with the 3C-like protease inhibitor GC376 on the treatment of naturally transmitted feline infectious peritonitis.Front Vet Sci. 2022 Oct 28;9:1002488. doi: 10.3389/fvets.2022.1002488. eCollection 2022. Front Vet Sci. 2022. PMID: 36387398 Free PMC article.
References
-
- Benlhassan-Chahour, K., C. Penit, V. Dioszeghy, F. Vasseur, G. Janvier, Y. Riviere, N. Dereuddre-Bosquet, D. Dormont, R. Le Grand, and B. Vaslin. 2003. Kinetics of lymphocyte proliferation during primary immune response in macaques infected with pathogenic simian immunodeficiency virus SIVmac251: preliminary report of the effect of early antiviral therapy. J. Virol. 77:12479-12493. - PMC - PubMed
-
- Bergmann, C. C., J. D. Altman, D. Hinton, and S. A. Stohlman. 1999. Inverted immunodominance and impaired cytolytic function of CD8+ T cells during viral persistence in the central nervous system. J. Immunol. 163:3379-3387. - PubMed
-
- Booth, C. M., L. M. Matukas, G. A. Tomlinson, A. R. Rachlis, D. B. Rose, H. A. Dwosh, S. L. Walmsley, T. Mazzulli, M. Avendano, P. Derkach, I. E. Ephtimios, I. Kitai, B. D. Mederski, S. B. Shadowitz, W. L. Gold, L. A. Hawryluck, E. Rea, J. S. Chenkin, D. W. Cescon, S. M. Poutanen, and A. S. Detsky. 2003. Clinical features and short-term outcomes of 144 patients with SARS in the greater Toronto area. JAMA 289:2801-2809. - PubMed
-
- Brown, T. D. K., and I. Brierly. 1995. The coronaviral nonstructural proteins, p. 191-217. In S. G. Siddell (ed.), The Coronaviridae. Plenum Press, New York, N.Y.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

