Mutations conferring resistance to human immunodeficiency virus type 1 fusion inhibitors are restricted by gp41 and Rev-responsive element functions
- PMID: 15613304
- PMCID: PMC538534
- DOI: 10.1128/JVI.79.2.764-770.2005
Mutations conferring resistance to human immunodeficiency virus type 1 fusion inhibitors are restricted by gp41 and Rev-responsive element functions
Abstract
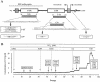
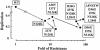
One of the human immunodeficiency virus (HIV) envelope proteins, gp41, plays a key role in HIV fusion. A gp41-derived peptide, T-20, efficiently inhibits HIV fusion and is currently approved for treatment of HIV-infected individuals. Although resistant variants have been reported, the mechanism of the resistance remains to be defined. To elucidate the mechanism in detail, we generated variants resistant to C34, a peptide derived from the gp41 carboxyl terminus heptad repeat (C-HR) in vitro. The resistant variants had a 5-amino-acid deletion in gp120 and a total of seven amino acid substitutions in gp41. Binding assays revealed that an I37K substitution in the N-terminal heptad repeat (N-HR) impaired the binding of C34, whereas an N126K substitution in the C-HR enhanced the binding to mutated N-HR, indicating that both mutations were directly involved in resistance. On the other hand, substitutions for A30 and D36 seemed to be secondary mutations, located complementary to each other in the Rev-responsive element (RRE), and were mutated simultaneously to maintain the secondary structure of the RRE that was impaired by the mutations at I37. Thus, HIV acquired resistance to C34 by mutations in N-HR, which directly interacted with C34. However, since this region also encoded the RRE, additional mutations were required to maintain viral replication. These results suggest that HIV fusion is one of the attractive targets for HIV chemotherapy.
Figures
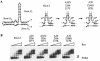
Similar articles
-
Characterization of HIV-1 resistance to a fusion inhibitor, N36, derived from the gp41 amino-terminal heptad repeat.Antiviral Res. 2010 Aug;87(2):179-86. doi: 10.1016/j.antiviral.2010.04.011. Epub 2010 May 8. Antiviral Res. 2010. PMID: 20438763
-
The stability of the intact envelope glycoproteins is a major determinant of sensitivity of HIV/SIV to peptidic fusion inhibitors.J Mol Biol. 2004 Jun 25;340(1):9-14. doi: 10.1016/j.jmb.2004.04.027. J Mol Biol. 2004. PMID: 15184018
-
Differential inhibition of HIV-1 and SIV envelope-mediated cell fusion by C34 peptides derived from the C-terminal heptad repeat of gp41 from diverse strains of HIV-1, HIV-2, and SIV.J Med Chem. 2005 Apr 21;48(8):3036-44. doi: 10.1021/jm049026h. J Med Chem. 2005. PMID: 15828842
-
HIV-1 gp41: mediator of fusion and target for inhibition.AIDS Rev. 2003 Oct-Dec;5(4):214-21. AIDS Rev. 2003. PMID: 15012000 Review.
-
HIV fusion inhibitors.Rev Med Virol. 2010 Jan;20(1):23-33. doi: 10.1002/rmv.631. Rev Med Virol. 2010. PMID: 19827030 Review.
Cited by
-
Origins of resistance to the HIVgp41 viral entry inhibitor T20.Biochemistry. 2010 May 4;49(17):3575-92. doi: 10.1021/bi901915g. Biochemistry. 2010. PMID: 20230061 Free PMC article.
-
Molecular mechanism of HIV-1 resistance to sifuvirtide, a clinical trial-approved membrane fusion inhibitor.J Biol Chem. 2018 Aug 17;293(33):12703-12718. doi: 10.1074/jbc.RA118.003538. Epub 2018 Jun 21. J Biol Chem. 2018. PMID: 29929981 Free PMC article.
-
Functional analyses reveal extensive RRE plasticity in primary HIV-1 sequences selected under selective pressure.PLoS One. 2014 Aug 29;9(8):e106299. doi: 10.1371/journal.pone.0106299. eCollection 2014. PLoS One. 2014. PMID: 25170621 Free PMC article.
-
Anti-HIV-1 activity determined by β-galactosidase activity in the multinuclear activation of an indicator assay is comparable with that by a conventional focus counting method.Antivir Chem Chemother. 2015 Apr;24(2):77-82. doi: 10.1177/2040206615614164. Epub 2015 Nov 2. Antivir Chem Chemother. 2015. PMID: 26527820 Free PMC article.
-
Impact of the HIV-1 env genetic context outside HR1-HR2 on resistance to the fusion inhibitor enfuvirtide and viral infectivity in clinical isolates.PLoS One. 2011;6(7):e21535. doi: 10.1371/journal.pone.0021535. Epub 2011 Jul 8. PLoS One. 2011. PMID: 21760896 Free PMC article.
References
-
- Chan, D. C., D. Fass, J. M. Berger, and P. S. Kim. 1997. Core structure of gp41 from the HIV envelope glycoprotein. Cell 89:263-273. - PubMed
-
- Derdeyn, C. A., J. M. Decker, J. N. Sfakianos, X. Wu, W. A. O'Brien, L. Ratner, J. C. Kappes, G. M. Shaw, and E. Hunter. 2000. Sensitivity of human immunodeficiency virus type 1 to the fusion inhibitor T-20 is modulated by coreceptor specificity defined by the V3 loop of gp120. J. Virol. 74:8358-8367. - PMC - PubMed
-
- Derdeyn, C. A., J. M. Decker, J. N. Sfakianos, Z. Zhang, W. A. O'Brien, L. Ratner, G. M. Shaw, and E. Hunter. 2001. Sensitivity of human immunodeficiency virus type 1 to fusion inhibitors targeted to the gp41 first heptad repeat involves distinct regions of gp41 and is consistently modulated by gp120 interactions with the coreceptor. J. Virol. 75:8605-8614. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

