Persistence of apoptotic cells without autoimmune disease or inflammation in CD14-/- mice
- PMID: 15611337
- PMCID: PMC2172617
- DOI: 10.1083/jcb.200410057
Persistence of apoptotic cells without autoimmune disease or inflammation in CD14-/- mice
Abstract
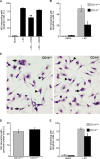
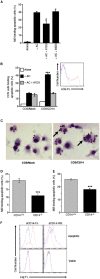
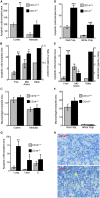
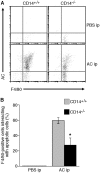
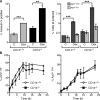
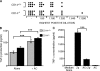
Interaction of macrophages with apoptotic cells involves multiple steps including recognition, tethering, phagocytosis, and anti-inflammatory macrophage responses. Defective apoptotic cell clearance is associated with pathogenesis of autoimmune disease. CD14 is a surface receptor that functions in vitro in the removal of apoptotic cells by human and murine macrophages, but its mechanism of action has not been defined. Here, we demonstrate that CD14 functions as a macrophage tethering receptor for apoptotic cells. Significantly, CD14(-/-) macrophages in vivo are defective in clearing apoptotic cells in multiple tissues, suggesting a broad role for CD14 in the clearance process. However, the resultant persistence of apoptotic cells does not lead to inflammation or increased autoantibody production, most likely because, as we show, CD14(-/-) macrophages retain the ability to generate anti-inflammatory signals in response to apoptotic cells. We conclude that CD14 plays a broad tethering role in apoptotic cell clearance in vivo and that apoptotic cells can persist in the absence of proinflammatory consequences.
Figures
Similar articles
-
Human CD14 mediates recognition and phagocytosis of apoptotic cells.Nature. 1998 Apr 2;392(6675):505-9. doi: 10.1038/33169. Nature. 1998. PMID: 9548256
-
Macrophage chemotaxis to apoptotic Burkitt's lymphoma cells in vitro: role of CD14 and CD36.Immunobiology. 2004;209(1-2):21-30. doi: 10.1016/j.imbio.2004.02.001. Immunobiology. 2004. PMID: 15481137
-
CD14 is a component of multiple recognition systems used by macrophages to phagocytose apoptotic lymphocytes.Cell Death Differ. 1999 Jun;6(6):583-92. doi: 10.1038/sj.cdd.4400529. Cell Death Differ. 1999. PMID: 10381656
-
The macrophage and the apoptotic cell: an innate immune interaction viewed simplistically?Immunology. 2004 Sep;113(1):1-14. doi: 10.1111/j.1365-2567.2004.01959.x. Immunology. 2004. PMID: 15312130 Free PMC article. Review.
-
Non-inflammatory/anti-inflammatory CD14 responses: CD14 in apoptosis.Chem Immunol. 2000;74:122-40. Chem Immunol. 2000. PMID: 10608085 Review. No abstract available.
Cited by
-
NETs: the missing link between cell death and systemic autoimmune diseases?Front Immunol. 2013 Jan 17;3:428. doi: 10.3389/fimmu.2012.00428. eCollection 2012. Front Immunol. 2013. PMID: 23335928 Free PMC article.
-
T cell targeting and phagocytosis of apoptotic biliary epithelial cells in primary biliary cirrhosis.J Autoimmun. 2006 Dec;27(4):232-41. doi: 10.1016/j.jaut.2006.11.004. Epub 2007 Jan 10. J Autoimmun. 2006. PMID: 17222534 Free PMC article.
-
Emerging and Novel Functions of Complement Protein C1q.Front Immunol. 2015 Jun 29;6:317. doi: 10.3389/fimmu.2015.00317. eCollection 2015. Front Immunol. 2015. PMID: 26175731 Free PMC article. Review.
-
The role of defective clearance of apoptotic cells in systemic autoimmunity.Nat Rev Rheumatol. 2010 May;6(5):280-9. doi: 10.1038/nrrheum.2010.46. Nat Rev Rheumatol. 2010. PMID: 20431553 Review.
-
Iron Handling in Tumor-Associated Macrophages-Is There a New Role for Lipocalin-2?Front Immunol. 2017 Sep 20;8:1171. doi: 10.3389/fimmu.2017.01171. eCollection 2017. Front Immunol. 2017. PMID: 28979267 Free PMC article. Review.
References
-
- Albert, M.L. 2004. Death-defying immunity: do apoptotic cells influence antigen processing and presentation? Nat. Rev. Immunol. 4:223–231. - PubMed
-
- Ayala, A., C.D. Herdon, D.L. Lehman, C.A. Ayala, and I.H. Chaudry. 1996. Differential induction of apoptosis in lymphoid tissues during sepsis: variation in onset, frequency, and the nature of the mediators. Blood. 87:4261–4275. - PubMed
-
- Blander, J.M., and R. Medzhitov. 2004. Regulation of phagosome maturation by signals from toll-like receptors. Science. 304:1014–1018. - PubMed
-
- Botto, M., C. DellAgnola, A.E. Bygrave, E.M. Thompson, H.T. Cook, F. Petry, M. Loos, P.P. Pandolfi, and M.J. Walport. 1998. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nat. Genet. 19:56–59. - PubMed
-
- Brown, S., I. Heinisch, E. Ross, K. Shaw, C.D. Buckley, and J. Savill. 2002. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature. 418:200–203. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

