The interplay of osteogenesis and hematopoiesis: expression of a constitutively active PTH/PTHrP receptor in osteogenic cells perturbs the establishment of hematopoiesis in bone and of skeletal stem cells in the bone marrow
- PMID: 15611335
- PMCID: PMC2172616
- DOI: 10.1083/jcb.200408079
The interplay of osteogenesis and hematopoiesis: expression of a constitutively active PTH/PTHrP receptor in osteogenic cells perturbs the establishment of hematopoiesis in bone and of skeletal stem cells in the bone marrow
Abstract
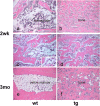
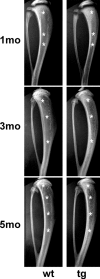
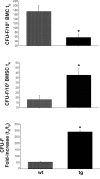
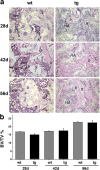
The ontogeny of bone marrow and its stromal compartment, which is generated from skeletal stem/progenitor cells, was investigated in vivo and ex vivo in mice expressing constitutively active parathyroid hormone/parathyroid hormone-related peptide receptor (PTH/PTHrP; caPPR) under the control of the 2.3-kb bone-specific mouse Col1A1 promoter/enhancer. The transgene promoted increased bone formation within prospective marrow space, but delayed the transition from bone to bone marrow during growth, the formation of marrow cavities, and the appearance of stromal cell types such as marrow adipocytes and cells supporting hematopoiesis. This phenotype resolved spontaneously over time, leading to the establishment of marrow containing a greatly reduced number of clonogenic stromal cells. Proliferative osteoprogenitors, but not multipotent skeletal stem cells (mesenchymal stem cells), capable of generating a complete heterotopic bone organ upon in vivo transplantation were assayable in the bone marrow of caPPR mice. Thus, PTH/PTHrP signaling is a major regulator of the ontogeny of the bone marrow and its stromal tissue, and of the skeletal stem cell compartment.
Figures



Similar articles
-
Constitutively active PTH/PTHrP receptor specifically expressed in osteoblasts enhances bone formation induced by bone marrow ablation.J Cell Physiol. 2012 Feb;227(2):408-15. doi: 10.1002/jcp.22986. J Cell Physiol. 2012. PMID: 21866553 Free PMC article.
-
Endogenous parathyroid hormone-related protein compensates for the absence of parathyroid hormone in promoting bone accrual in vivo in a model of bone marrow ablation.J Bone Miner Res. 2013 Sep;28(9):1898-911. doi: 10.1002/jbmr.2000. J Bone Miner Res. 2013. PMID: 23716486
-
Constitutively active parathyroid hormone receptor signaling in cells in osteoblastic lineage suppresses mechanical unloading-induced bone resorption.J Biol Chem. 2007 Aug 31;282(35):25509-16. doi: 10.1074/jbc.M610782200. Epub 2007 May 11. J Biol Chem. 2007. PMID: 17500070 Free PMC article.
-
Current perspectives on parathyroid hormone (PTH) and PTH-related protein (PTHrP) as bone anabolic therapies.Biochem Pharmacol. 2013 May 15;85(10):1417-23. doi: 10.1016/j.bcp.2013.03.002. Epub 2013 Mar 13. Biochem Pharmacol. 2013. PMID: 23500550 Review.
-
PTH and PTHrP signaling in osteoblasts.Cell Signal. 2009 Aug;21(8):1245-54. doi: 10.1016/j.cellsig.2009.02.012. Epub 2009 Feb 26. Cell Signal. 2009. PMID: 19249350 Free PMC article. Review.
Cited by
-
Skeletal stem cells.Development. 2015 Mar 15;142(6):1023-7. doi: 10.1242/dev.102210. Development. 2015. PMID: 25758217 Free PMC article. Review.
-
Cyclic AMP signaling in bone marrow stromal cells has reciprocal effects on the ability of mesenchymal stem cells to differentiate into mature osteoblasts versus mature adipocytes.Endocrine. 2012 Dec;42(3):622-36. doi: 10.1007/s12020-012-9717-9. Epub 2012 Jun 14. Endocrine. 2012. PMID: 22695986 Free PMC article.
-
Constitutively active PTH/PTHrP receptor specifically expressed in osteoblasts enhances bone formation induced by bone marrow ablation.J Cell Physiol. 2012 Feb;227(2):408-15. doi: 10.1002/jcp.22986. J Cell Physiol. 2012. PMID: 21866553 Free PMC article.
-
Osteopontin negatively regulates parathyroid hormone receptor signaling in osteoblasts.J Biol Chem. 2008 Jul 11;283(28):19400-9. doi: 10.1074/jbc.M800005200. Epub 2008 Apr 16. J Biol Chem. 2008. PMID: 18417476 Free PMC article.
-
Osteoblast expression of an engineered Gs-coupled receptor dramatically increases bone mass.Proc Natl Acad Sci U S A. 2008 Jan 29;105(4):1209-14. doi: 10.1073/pnas.0707457105. Epub 2008 Jan 22. Proc Natl Acad Sci U S A. 2008. PMID: 18212126 Free PMC article.
References
-
- Begley, C.T., M.J. Doherty, D.P. Hankey, and D.J. Wilson. 1993. The culture of human osteoblasts upon bone graft substitutes. Bone. 14:661–666. - PubMed
-
- Bianco, P., and E. Bonucci. 1991. Endosteal surfaces in hyperparathyroidism: an enzyme cytochemical study on low-temperature-processed, glycol-methacrylate-embedded bone biopsies. Virchows Arch. A Pathol. Anat. Histopathol. 419:425–431. - PubMed
-
- Bianco, P., and A. Boyde. 1993. Confocal images of marrow stromal (Westen-Bainton) cells. Histochemistry. 100:93–99. - PubMed
-
- Bianco, P., and M. Riminucci. 1998. The bone marrow stroma in vivo: ontogeny, structure, cellular composition and changes in disease. Marrow Stromal Cell Culture. M.A. Owen and J.N. Beresford, editors. Cambridge University Press, Cambridge, UK. 10–25.
-
- Bianco, P., and P. Robey. 1999. Diseases of bone and the stromal cell lineage. J. Bone Miner. Res. 14:336–341. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

