Structure of a proteolytically resistant core from the severe acute respiratory syndrome coronavirus S2 fusion protein
- PMID: 15604146
- PMCID: PMC539766
- DOI: 10.1073/pnas.0406128102
Structure of a proteolytically resistant core from the severe acute respiratory syndrome coronavirus S2 fusion protein
Abstract
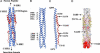
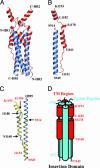
A coronavirus (CoV) has recently been identified as the causative agent of the severe acute respiratory syndrome (SARS) in humans. CoVs enter target cells through fusion of viral and cellular membranes mediated by the viral envelope glycoprotein S. We have determined by x-ray crystallography the structure of a proteolytically stable core fragment from the heptad repeat (HR) regions HR1 and HR2 of the SARS-CoV S protein. We have also determined the structure of an HR1-HR2 S core fragment, containing a shorter HR1 peptide and a C-terminally longer HR2 peptide that extends up to the transmembrane region. In these structures, three HR1 helices form a parallel coiled-coil trimer, whereas three HR2 peptides pack in an oblique and antiparallel fashion into the coiled-coil hydrophobic grooves, adopting mixed extended and alpha-helical conformations as in postfusion paramyxoviruses F proteins structures. Our structure positions a previously proposed internal fusion peptide adjacent to the N-terminus of HR1. Peptides from the HR2 region of SARS-CoV S have been shown to inhibit viral entry and infection in vitro. The structures presented here can thus open the path to the design of small-molecule inhibitors of viral entry and candidate vaccine antigens against this virus.
Figures

Similar articles
-
Structural basis for coronavirus-mediated membrane fusion. Crystal structure of mouse hepatitis virus spike protein fusion core.J Biol Chem. 2004 Jul 16;279(29):30514-22. doi: 10.1074/jbc.M403760200. Epub 2004 Apr 27. J Biol Chem. 2004. PMID: 15123674 Free PMC article.
-
Crystal structure of severe acute respiratory syndrome coronavirus spike protein fusion core.J Biol Chem. 2004 Nov 19;279(47):49414-9. doi: 10.1074/jbc.M408782200. Epub 2004 Sep 1. J Biol Chem. 2004. PMID: 15345712 Free PMC article.
-
Interaction between heptad repeat 1 and 2 regions in spike protein of SARS-associated coronavirus: implications for virus fusogenic mechanism and identification of fusion inhibitors.Lancet. 2004 Mar 20;363(9413):938-47. doi: 10.1016/S0140-6736(04)15788-7. Lancet. 2004. PMID: 15043961 Free PMC article.
-
Severe acute respiratory syndrome coronavirus entry into host cells: Opportunities for therapeutic intervention.Med Res Rev. 2006 Jul;26(4):414-33. doi: 10.1002/med.20055. Med Res Rev. 2006. PMID: 16521129 Free PMC article. Review.
-
The SARS-CoV S glycoprotein.Cell Mol Life Sci. 2004 Oct;61(19-20):2428-30. doi: 10.1007/s00018-004-4257-y. Cell Mol Life Sci. 2004. PMID: 15526150 Free PMC article. Review.
Cited by
-
Crystal Structures of Fusion Cores from CCoV-HuPn-2018 and SADS-CoV.Viruses. 2024 Feb 9;16(2):272. doi: 10.3390/v16020272. Viruses. 2024. PMID: 38400047 Free PMC article.
-
Characterization of monoclonal antibodies against porcine epidemic diarrhea virus S1/S2 junction protein.AMB Express. 2023 Jul 12;13(1):74. doi: 10.1186/s13568-023-01573-4. AMB Express. 2023. PMID: 37436550 Free PMC article.
-
SARS-CoV-2 S Glycoprotein Stabilization Strategies.Viruses. 2023 Feb 17;15(2):558. doi: 10.3390/v15020558. Viruses. 2023. PMID: 36851772 Free PMC article. Review.
-
Structural conservation among variants of the SARS-CoV-2 spike postfusion bundle.Proc Natl Acad Sci U S A. 2022 Apr 19;119(16):e2119467119. doi: 10.1073/pnas.2119467119. Epub 2022 Apr 1. Proc Natl Acad Sci U S A. 2022. PMID: 35363556 Free PMC article.
-
Spike Glycoprotein Is Central to Coronavirus Pathogenesis-Parallel Between m-CoV and SARS-CoV-2.Ann Neurosci. 2021 Jul;28(3-4):201-218. doi: 10.1177/09727531211023755. Epub 2021 Oct 12. Ann Neurosci. 2021. PMID: 35341224 Free PMC article. Review.
References
-
- Holmes, K. V. (2001) Fields Virology (Lippincott, Williams and Wilkins, New York).
-
- Lai, M. M. C. & Holmes, K. V. (2001) Fields Virology (Lippincott, Williams and Wilkins, New York).
-
- Peiris, J. S., Yuen, K. Y., Osterhaus, A. D. & Stohr, K. (2003) N. Engl. J. Med. 349, 2431–2441. - PubMed
-
- Ksiazek, T. G., Erdman, D., Goldsmith, C. S., Zaki, S. R., Peret, T., Emery, S., Tong, S., Urbani, C., Comer, J. A., Lim, W., et al. (2003) N. Engl. J. Med. 348, 1953–1966. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

