Lentivector-mediated SMN replacement in a mouse model of spinal muscular atrophy
- PMID: 15599397
- PMCID: PMC535071
- DOI: 10.1172/JCI22922
Lentivector-mediated SMN replacement in a mouse model of spinal muscular atrophy
Abstract
Spinal muscular atrophy (SMA) is a frequent recessive autosomal disorder. It is caused by mutations or deletion of the telomeric copy of the survival motor neuron (SMN) gene, leading to depletion in SMN protein levels. The treatment rationale for SMA is to halt or delay the degeneration of motor neurons, but to date there are no effective drug treatments for this disease. We have previously demonstrated that pseudotyping of the nonprimate equine infectious anemia virus (using the lentivector gene transfer system) with the glycoprotein of the Evelyn-Rokitnicki-Abelseth strain of the rabies virus confers retrograde axonal transport on these vectors. Here, we report that lentivector expressing human SMN was successfully used to restore SMN protein levels in SMA type 1 fibroblasts. Multiple single injections of a lentiviral vector expressing SMN in various muscles of SMA mice restored SMN to motor neurons, reduced motor neuron death, and increased the life expectancy by an average of 3 and 5 days (20% and 38%) compared with LacZ and untreated animals, respectively. Further extension of survival by SMN expression constructs will likely require a knowledge of when and/or where high levels of SMN are needed.
Figures
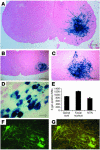
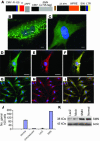
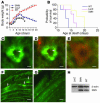
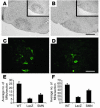

Similar articles
-
Reduced survival motor neuron (Smn) gene dose in mice leads to motor neuron degeneration: an animal model for spinal muscular atrophy type III.Hum Mol Genet. 2000 Feb 12;9(3):341-6. doi: 10.1093/hmg/9.3.341. Hum Mol Genet. 2000. PMID: 10655542
-
A transgene carrying an A2G missense mutation in the SMN gene modulates phenotypic severity in mice with severe (type I) spinal muscular atrophy.J Cell Biol. 2003 Jan 6;160(1):41-52. doi: 10.1083/jcb.200208079. Epub 2003 Jan 6. J Cell Biol. 2003. PMID: 12515823 Free PMC article.
-
Therapeutics development for spinal muscular atrophy.NeuroRx. 2006 Apr;3(2):235-45. doi: 10.1016/j.nurx.2006.01.010. NeuroRx. 2006. PMID: 16554261 Free PMC article. Review.
-
Development of a gene therapy strategy for the restoration of survival motor neuron protein expression: implications for spinal muscular atrophy therapy.Hum Gene Ther. 2003 Jan 20;14(2):179-88. doi: 10.1089/104303403321070874. Hum Gene Ther. 2003. PMID: 12614569
-
Congenital bone fractures in spinal muscular atrophy: functional role for SMN protein in bone remodeling.J Child Neurol. 2007 Aug;22(8):967-73. doi: 10.1177/0883073807305664. J Child Neurol. 2007. PMID: 17761651 Free PMC article. Review.
Cited by
-
Altering the tropism of lentiviral vectors through pseudotyping.Curr Gene Ther. 2005 Aug;5(4):387-98. doi: 10.2174/1566523054546224. Curr Gene Ther. 2005. PMID: 16101513 Free PMC article. Review.
-
Translating SOD1 Gene Silencing toward the Clinic: A Highly Efficacious, Off-Target-free, and Biomarker-Supported Strategy for fALS.Mol Ther Nucleic Acids. 2018 Sep 7;12:75-88. doi: 10.1016/j.omtn.2018.04.015. Epub 2018 May 3. Mol Ther Nucleic Acids. 2018. PMID: 30195799 Free PMC article.
-
Delivery of bifunctional RNAs that target an intronic repressor and increase SMN levels in an animal model of spinal muscular atrophy.Hum Mol Genet. 2009 May 1;18(9):1600-11. doi: 10.1093/hmg/ddp076. Epub 2009 Feb 19. Hum Mol Genet. 2009. PMID: 19228773 Free PMC article.
-
Bifunctional RNAs targeting the intronic splicing silencer N1 increase SMN levels and reduce disease severity in an animal model of spinal muscular atrophy.Mol Ther. 2012 Jan;20(1):119-26. doi: 10.1038/mt.2011.232. Epub 2011 Oct 25. Mol Ther. 2012. PMID: 22031236 Free PMC article.
-
Modeling spinal muscular atrophy in Drosophila links Smn to FGF signaling.J Cell Biol. 2011 Feb 7;192(3):481-95. doi: 10.1083/jcb.201004016. J Cell Biol. 2011. PMID: 21300852 Free PMC article.
References
-
- Gendron NH, MacKenzie AE. Spinal muscular atrophy: molecular pathophysiology. Curr. Opin. Neurol. 1999;12:137–142. - PubMed
-
- Sendtner M. Molecular mechanisms in spinal muscular atrophy: models and perspectives. Curr. Opin. Neurol. 2001;14:629–634. - PubMed
-
- Lefebvre S, et al. Identification and characterization of a spinal muscular atrophy-determining gene. Cell. 1995;80:155–165. - PubMed
-
- Munsat TL, Davies KE. International SMA consortium meeting. Neuromuscul. Disord. 1992;2:423–428. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

