Replication-dependent destruction of Cdt1 limits DNA replication to a single round per cell cycle in Xenopus egg extracts
- PMID: 15598982
- PMCID: PMC540230
- DOI: 10.1101/gad.1255805
Replication-dependent destruction of Cdt1 limits DNA replication to a single round per cell cycle in Xenopus egg extracts
Abstract
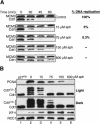
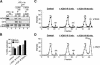
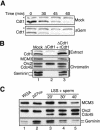
In eukaryotes, prereplication complexes (pre-RCs) containing ORC, Cdc6, Cdt1, and MCM2-7 are assembled on chromatin in the G1 phase. In S phase, when DNA replication initiates, pre-RCs are disassembled, and new pre-RC assembly is restricted until the following G1 period. As a result, DNA replication is limited to a single round per cell cycle. One inhibitor of pre-RC assembly, geminin, was discovered in Xenopus, and it binds and inactivates Cdt1 in S phase. However, removal of geminin from Xenopus egg extracts is insufficient to cause rereplication, suggesting that other safeguards against rereplication exist. Here, we show that Cdt1 is completely degraded by ubiquitin-mediated proteolysis during the course of the first round of DNA replication in Xenopus egg extracts. Degradation depends on Cdk2/Cyclin E, Cdc45, RPA, and polymerase alpha, demonstrating a requirement for replication initiation. Cdt1 is ubiquitinated on chromatin, and this process also requires replication initiation. Once replication has initiated, Cdk2/Cyclin E is dispensable for Cdt1 degradation. When fresh Cdt1 is supplied after the first round of DNA replication, significant rereplication results, and rereplication is enhanced in the absence of geminin. Our results identify a replication-dependent proteolytic pathway that targets Cdt1 and that acts redundantly with geminin to inactivate Cdt1 in S phase.
Figures




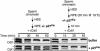
Similar articles
-
A Cdt1-geminin complex licenses chromatin for DNA replication and prevents rereplication during S phase in Xenopus.EMBO J. 2006 Dec 13;25(24):5764-74. doi: 10.1038/sj.emboj.7601436. Epub 2006 Nov 23. EMBO J. 2006. PMID: 17124498 Free PMC article.
-
Licensing for DNA replication requires a strict sequential assembly of Cdc6 and Cdt1 onto chromatin in Xenopus egg extracts.Nucleic Acids Res. 2005 Feb 1;33(2):765-75. doi: 10.1093/nar/gki226. Print 2005. Nucleic Acids Res. 2005. PMID: 15687385 Free PMC article.
-
Cell cycle-dependent regulation of the association between origin recognition proteins and somatic cell chromatin.EMBO J. 2002 Mar 15;21(6):1437-46. doi: 10.1093/emboj/21.6.1437. EMBO J. 2002. PMID: 11889049 Free PMC article.
-
Cdt1 and geminin: role during cell cycle progression and DNA damage in higher eukaryotes.Front Biosci. 2007 Jan 1;12:1629-41. doi: 10.2741/2175. Front Biosci. 2007. PMID: 17127409 Review.
-
Geminin-Cdt1 balance is critical for genetic stability.Mutat Res. 2005 Jan 6;569(1-2):111-21. doi: 10.1016/j.mrfmmm.2004.05.026. Mutat Res. 2005. PMID: 15603756 Review.
Cited by
-
Activation of a human chromosomal replication origin by protein tethering.Nucleic Acids Res. 2013 Jul;41(13):6460-74. doi: 10.1093/nar/gkt368. Epub 2013 May 8. Nucleic Acids Res. 2013. PMID: 23658226 Free PMC article.
-
Single-cell transcriptome analysis defines heterogeneity of the murine pancreatic ductal tree.Elife. 2021 May 19;10:e67776. doi: 10.7554/eLife.67776. Elife. 2021. PMID: 34009124 Free PMC article.
-
Cdt1 degradation to prevent DNA re-replication: conserved and non-conserved pathways.Cell Div. 2007 Jun 12;2:18. doi: 10.1186/1747-1028-2-18. Cell Div. 2007. PMID: 17565698 Free PMC article.
-
Cdt1 proteolysis is promoted by dual PIP degrons and is modulated by PCNA ubiquitylation.Nucleic Acids Res. 2011 Aug;39(14):5978-90. doi: 10.1093/nar/gkr222. Epub 2011 Apr 14. Nucleic Acids Res. 2011. PMID: 21493688 Free PMC article.
-
Dynamic interactions of high Cdt1 and geminin levels regulate S phase in early Xenopus embryos.Development. 2012 Jan;139(1):63-74. doi: 10.1242/dev.068676. Epub 2011 Nov 17. Development. 2012. PMID: 22096080 Free PMC article.
References
-
- Abraham R.T. 2001. Cell cycle checkpoint signaling through the ATM and ATR kinases. Genes & Dev. 15: 2177–2196. - PubMed
-
- Bates S., Ryan, K.M., Phillips, A.C., and Vousden, K.H. 1998. Cell cycle arrest and DNA endoreduplication following p21Waf1/Cip1 expression. Oncogene 17: 1691–1703. - PubMed
-
- Bell S.P. and Dutta, A. 2002. DNA replication in eukaryotic cells. Annu. Rev. Biochem. 71: 333–374. - PubMed
-
- Blow J.J. and Laskey, R.A. 1986. Initiation of DNA replication in nuclei and purified DNA by a cell-free extract of Xenopus eggs. Cell 47: 577–587. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
