Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages
- PMID: 15595946
- PMCID: PMC2690188
- DOI: 10.1111/j.0887-378X.2004.00327.x
Evidence-based medicine, heterogeneity of treatment effects, and the trouble with averages
Erratum in
- Milbank Q. 2006;84(4):759-60
Abstract
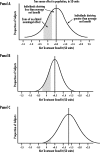
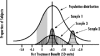
Evidence-based medicine is the application of scientific evidence to clinical practice. This article discusses the difficulties of applying global evidence ("average effects" measured as population means) to local problems (individual patients or groups who might depart from the population average). It argues that the benefit or harm of most treatments in clinical trials can be misleading and fail to reveal the potentially complex mixture of substantial benefits for some, little benefit for many, and harm for a few. Heterogeneity of treatment effects reflects patient diversity in risk of disease, responsiveness to treatment, vulnerability to adverse effects, and utility for different outcomes. Recognizing these factors, researchers can design studies that better characterize who will benefit from medical treatments, and clinicians and policymakers can make better use of the results.
Figures

Similar articles
-
The future of Cochrane Neonatal.Early Hum Dev. 2020 Nov;150:105191. doi: 10.1016/j.earlhumdev.2020.105191. Epub 2020 Sep 12. Early Hum Dev. 2020. PMID: 33036834
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Do Limitations in the Design of PARADIGM-HF Justify the Slow Real World Uptake of Sacubitril/Valsartan (Entresto)?Cardiovasc Drugs Ther. 2018 Dec;32(6):633-635. doi: 10.1007/s10557-018-6830-x. Cardiovasc Drugs Ther. 2018. PMID: 30232657 No abstract available.
-
Evidence Brief: The Quality of Care Provided by Advanced Practice Nurses [Internet].Washington (DC): Department of Veterans Affairs (US); 2014 Sep. Washington (DC): Department of Veterans Affairs (US); 2014 Sep. PMID: 27606392 Free Books & Documents. Review.
-
Screening for Cognitive Impairment in Older Adults: An Evidence Update for the U.S. Preventive Services Task Force [Internet].Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Feb. Report No.: 19-05257-EF-1. Rockville (MD): Agency for Healthcare Research and Quality (US); 2020 Feb. Report No.: 19-05257-EF-1. PMID: 32129963 Free Books & Documents. Review.
Cited by
-
Heterogeneity of treatment effects: from "How to treat" to "Whom to treat".Evid Based Spine Care J. 2011 May;2(2):7-10. doi: 10.1055/s-0030-1267099. Evid Based Spine Care J. 2011. PMID: 23637676 Free PMC article. No abstract available.
-
Providing Emotional Support During the Process of Multiple Sclerosis Diagnosis (PrEliMS): A Feasibility Randomised Controlled Trial.Clin Rehabil. 2024 Nov;38(11):1506-1520. doi: 10.1177/02692155241284781. Epub 2024 Sep 25. Clin Rehabil. 2024. PMID: 39318328 Free PMC article. Clinical Trial.
-
Making sense of mobile health data: an open architecture to improve individual- and population-level health.J Med Internet Res. 2012 Aug 9;14(4):e112. doi: 10.2196/jmir.2152. J Med Internet Res. 2012. PMID: 22875563 Free PMC article.
-
Understanding heterogeneity in response to antidiabetes treatment: a post hoc analysis using SIDES, a subgroup identification algorithm.J Diabetes Sci Technol. 2013 Mar 1;7(2):420-30. doi: 10.1177/193229681300700219. J Diabetes Sci Technol. 2013. PMID: 23567001 Free PMC article. Clinical Trial.
-
Emotional benefits of mindfulness-based stress reduction in older adults: the moderating roles of age and depressive symptom severity.Aging Ment Health. 2013;17(7):823-9. doi: 10.1080/13607863.2013.799118. Epub 2013 May 22. Aging Ment Health. 2013. PMID: 23697871 Free PMC article. Clinical Trial.
References
-
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, Cole B, Mott LA. The Relationship of APOE Genotype to Neuropsychological Performance in Long-Term Cancer Survivors Treated with Standard Dose Chemotherapy. Psycho-oncology. 2003;12:612–9. 10.1002/pon.742. - DOI - PubMed
-
- B-Blocker Heart Attack Trial Research Group. A Randomized Trial of Propranolol in Patients with Acute Myocardial Infarction. I. Mortality Results. Journal of the American Medical Association. 1982;247:1707–14. 10.1001/jama.247.12.1707. - DOI - PubMed
-
- Baird KL. The New NIH and FDA Medical Research Policies: Targeting Gender, Promoting Justice. Journal of Health Politics, Policy and Law. 1999;24(3):531–65. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

