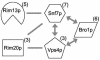
Snf7p, a component of the ESCRT-III protein complex, is an upstream member of the RIM101 pathway in Candida albicans
- PMID: 15590834
- PMCID: PMC539037
- DOI: 10.1128/EC.3.6.1609-1618.2004
Snf7p, a component of the ESCRT-III protein complex, is an upstream member of the RIM101 pathway in Candida albicans
Abstract
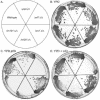
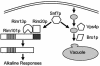
The success of Candida albicans as an opportunistic pathogen is based in part on its ability to adapt to diverse environments. The RIM101 pathway governs adaptation to neutral-alkaline environments and is required for virulence. Analysis of a genomic two-hybrid study conducted with Saccharomyces cerevisiae revealed that components involved in multivesicular bodies (MVB) transport may interact with RIM101 pathway members. Thus, we hypothesized that these proteins may function in the RIM101 pathway in C. albicans. We identified C. albicans homologs to S. cerevisiae Snf7p, Vps4p, and Bro1p and generated mutants in the cognate gene. We found that snf7Delta/Delta mutants, but not vps4Delta/Delta nor bro1Delta/Delta mutants, had phenotypes similar to, but more severe than, those of RIM101 pathway mutants. We found that the constitutively active RIM101-405 allele partially rescued snf7Delta/Delta mutant phenotypes. The vps4Delta/Delta mutant had subtle phenotypes, but these were not rescued by the RIM101-405 allele. Further, we found that the snf7Delta/Delta, vps4Delta/Delta, and bro1Delta/Delta mutants did not efficiently localize the vital dye FM4-64 to the vacuole and that it was often accumulated in an MVB-like compartment. This phenotype was not rescued by RIM101-405 or observed in RIM101 pathway mutants. These results suggest that Snf7p may serve two functions in the cell: one as a RIM101 pathway member and one for MVB transport to the vacuole.
Figures
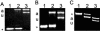





Similar articles
-
Multivesicular body-ESCRT components function in pH response regulation in Saccharomyces cerevisiae and Candida albicans.Mol Biol Cell. 2004 Dec;15(12):5528-37. doi: 10.1091/mbc.e04-08-0666. Epub 2004 Sep 15. Mol Biol Cell. 2004. PMID: 15371534 Free PMC article.
-
Deletions of endocytic components VPS28 and VPS32 affect growth at alkaline pH and virulence through both RIM101-dependent and RIM101-independent pathways in Candida albicans.Infect Immun. 2005 Dec;73(12):7977-87. doi: 10.1128/IAI.73.12.7977-7987.2005. Infect Immun. 2005. PMID: 16299290 Free PMC article.
-
The Candida albicans ESCRT pathway makes Rim101-dependent and -independent contributions to pathogenesis.Eukaryot Cell. 2010 Aug;9(8):1203-15. doi: 10.1128/EC.00056-10. Epub 2010 Jun 25. Eukaryot Cell. 2010. PMID: 20581294 Free PMC article.
-
[The response to environmental pH of RIM101 pathway in Candida albicans].Wei Sheng Wu Xue Bao. 2007 Apr;47(2):366-9. Wei Sheng Wu Xue Bao. 2007. PMID: 17552252 Review. Chinese.
-
Adaptation to environmental pH in Candida albicans and its relation to pathogenesis.Curr Genet. 2003 Oct;44(1):1-7. doi: 10.1007/s00294-003-0415-2. Epub 2003 Jun 18. Curr Genet. 2003. PMID: 12819929 Review.
Cited by
-
Evidence for novel pH-dependent regulation of Candida albicans Rim101, a direct transcriptional repressor of the cell wall beta-glycosidase Phr2.Eukaryot Cell. 2006 Sep;5(9):1550-9. doi: 10.1128/EC.00088-06. Eukaryot Cell. 2006. PMID: 16963637 Free PMC article.
-
Physiological involvement in pH signaling of Vps24-mediated recruitment of Aspergillus PalB cysteine protease to ESCRT-III.J Biol Chem. 2009 Feb 13;284(7):4404-12. doi: 10.1074/jbc.M808645200. Epub 2008 Dec 3. J Biol Chem. 2009. PMID: 19056728 Free PMC article.
-
Cryptococcus neoformans requires the ESCRT protein Vps23 for iron acquisition from heme, for capsule formation, and for virulence.Infect Immun. 2013 Jan;81(1):292-302. doi: 10.1128/IAI.01037-12. Epub 2012 Nov 6. Infect Immun. 2013. PMID: 23132495 Free PMC article.
-
Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans.Microbiol Mol Biol Rev. 2007 Jun;71(2):348-76. doi: 10.1128/MMBR.00009-06. Microbiol Mol Biol Rev. 2007. PMID: 17554048 Free PMC article. Review.
-
Recruitment of the ESCRT machinery to a putative seven-transmembrane-domain receptor is mediated by an arrestin-related protein.Mol Cell Biol. 2010 Feb;30(4):897-907. doi: 10.1128/MCB.00132-09. Epub 2009 Dec 22. Mol Cell Biol. 2010. PMID: 20028738 Free PMC article.
References
-
- Babst, M., D. J. Katzmann, E. J. Estepa-Sabal, T. Meerloo, and S. D. Emr. 2002. Escrt-III: an endosome-associated heterooligomeric protein complex required for mvb sorting. Dev. Cell 3:271-282. - PubMed
-
- Bensen, E. S., S. J. Martin, M. Li, J. Berman, and D. A. Davis. Transcriptional profiling in C. albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol. Microbiol., in press. - PubMed
-
- Bruckmann, A., W. Kunkel, K. Augsten, R. Wetzker, and R. Eck. 2001. The deletion of CaVPS34 in the human pathogenic yeast Candida albicans causes defects in vesicle-mediated protein sorting and nuclear segregation. Yeast 18:343-353. - PubMed
-
- Calderone, R. A. 2002. Candida and candidiasis. ASM Press, Washington, D.C.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

