Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p
- PMID: 15583031
- PMCID: PMC2172445
- DOI: 10.1083/jcb.200408124
Vesicles carry most exocyst subunits to exocytic sites marked by the remaining two subunits, Sec3p and Exo70p
Abstract
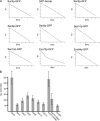
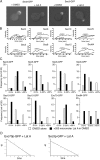
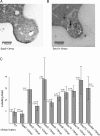
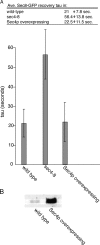
Exocytosis in the budding yeast Saccharomyces cerevisiae occurs at discrete domains of the plasma membrane. The protein complex that tethers incoming vesicles to sites of secretion is known as the exocyst. We have used photobleaching recovery experiments to characterize the dynamic behavior of the eight subunits that make up the exocyst. One subset (Sec5p, Sec6p, Sec8p, Sec10p, Sec15p, and Exo84p) exhibits mobility similar to that of the vesicle-bound Rab family protein Sec4p, whereas Sec3p and Exo70p exhibit substantially more stability. Disruption of actin assembly abolishes the ability of the first subset of subunits to recover after photobleaching, whereas Sec3p and Exo70p are resistant. Immunogold electron microscopy and epifluorescence video microscopy indicate that all exocyst subunits, except for Sec3p, are associated with secretory vesicles as they arrive at exocytic sites. Assembly of the exocyst occurs when the first subset of subunits, delivered on vesicles, joins Sec3p and Exo70p on the plasma membrane. Exocyst assembly serves to both target and tether vesicles to sites of exocytosis.
Figures


Similar articles
-
Two subunits of the exocyst, Sec3p and Exo70p, can function exclusively on the plasma membrane.Mol Biol Cell. 2018 Mar 15;29(6):736-750. doi: 10.1091/mbc.E17-08-0518. Epub 2018 Jan 17. Mol Biol Cell. 2018. PMID: 29343551 Free PMC article.
-
Functional specialization within a vesicle tethering complex: bypass of a subset of exocyst deletion mutants by Sec1p or Sec4p.J Cell Biol. 2004 Dec 6;167(5):875-87. doi: 10.1083/jcb.200408001. J Cell Biol. 2004. PMID: 15583030 Free PMC article.
-
An internal domain of Exo70p is required for actin-independent localization and mediates assembly of specific exocyst components.Mol Biol Cell. 2009 Jan;20(1):153-63. doi: 10.1091/mbc.e08-02-0157. Epub 2008 Oct 22. Mol Biol Cell. 2009. PMID: 18946089 Free PMC article.
-
The exocyst complex in polarized exocytosis.Int Rev Cytol. 2004;233:243-65. doi: 10.1016/S0074-7696(04)33006-8. Int Rev Cytol. 2004. PMID: 15037366 Review.
-
The exocyst meets the translocon: a regulatory circuit for secretion and protein synthesis?Trends Cell Biol. 2004 Feb;14(2):61-3. doi: 10.1016/j.tcb.2003.12.008. Trends Cell Biol. 2004. PMID: 15106610 Review.
Cited by
-
Exorcising the exocyst complex.Traffic. 2012 Jul;13(7):898-907. doi: 10.1111/j.1600-0854.2012.01353.x. Epub 2012 Apr 8. Traffic. 2012. PMID: 22420621 Free PMC article. Review.
-
Central roles of small GTPases in the development of cell polarity in yeast and beyond.Microbiol Mol Biol Rev. 2007 Mar;71(1):48-96. doi: 10.1128/MMBR.00028-06. Microbiol Mol Biol Rev. 2007. PMID: 17347519 Free PMC article. Review.
-
Spitzenkorper, exocyst, and polarisome components in Candida albicans hyphae show different patterns of localization and have distinct dynamic properties.Eukaryot Cell. 2010 Oct;9(10):1455-65. doi: 10.1128/EC.00109-10. Epub 2010 Aug 6. Eukaryot Cell. 2010. PMID: 20693302 Free PMC article.
-
Yarrowia lipolytica vesicle-mediated protein transport pathways.BMC Evol Biol. 2007 Nov 12;7:219. doi: 10.1186/1471-2148-7-219. BMC Evol Biol. 2007. PMID: 17997821 Free PMC article.
-
Vesicle docking to the spindle pole body is necessary to recruit the exocyst during membrane formation in Saccharomyces cerevisiae.Mol Biol Cell. 2010 Nov 1;21(21):3693-707. doi: 10.1091/mbc.E10-07-0563. Epub 2010 Sep 8. Mol Biol Cell. 2010. PMID: 20826607 Free PMC article.
References
-
- Amit, A.G., R.A. Mariuzza, S.E. Phillips, and R.J. Poljak. 1985. Three-dimensional structure of an antigen-antibody complex at 6 Å resolution. Nature. 313:156–158. - PubMed
-
- Andrews, H.K., Y.Q. Zhang, N. Trotta, and K. Broadie. 2002. Drosophila Sec10 is required for hormone secretion but not general exocytosis or neurotransmission. Traffic. 3:906–921. - PubMed
-
- Bielinski, D.F., H.Y. Pyun, K. Linko-Stentz, I.G. Macara, and R.E. Fine. 1993. Ral and Rab3a are major GTP-binding proteins of axonal rapid transport and synaptic vesicles and do not redistribute following depolarization stimulated synaptosomal exocytosis. Biochim. Biophys. Acta. 1151:246–256. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

