A novel cell-based binding assay system reconstituting interaction between SARS-CoV S protein and its cellular receptor
- PMID: 15582697
- PMCID: PMC7112911
- DOI: 10.1016/j.jviromet.2004.09.008
A novel cell-based binding assay system reconstituting interaction between SARS-CoV S protein and its cellular receptor
Abstract
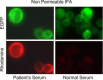
Severe acute respiratory syndrome (SARS), a life-threatening disease, is caused by the newly identified virus SARS coronavirus (SARS-CoV). In order to study the spike (S) protein of this highly contagious virus, we established a clonal cell-line, CHO-SG, from the Chinese hamster ovary cells that stably expresses C-terminally EGFP-tagged SARS-CoV S protein (S-EGFP). The ectodomain of the S glycoprotein is localized on the surface of CHO-SG cells with N-acetyl-glucosamine-terminated carbohydrate structure. CHO-SG cells associated tightly with Vero E6 cells, a SARS-CoV receptor (ACE2) expressing cell-line, and the interaction remained stable under highly stringent condition (1M NaCl). This interaction could be blocked by either the serum from a SARS convalescent patient or a goat anti-ACE2 antibody, indicating that the interaction is specific. A binding epitope with lesser degree of glycosylation and native conformation was localized by using rabbit anti-sera raised against five denatured recombinant S protein fragments expressed in Escherichia coli. One of the sera obtained from the fragment encompassing amino acids 48-358 significantly blocked the interaction between CHO-SG and Vero E6 cells. The region is useful for studying neutralizing antibodies in future vaccine development. This paper describes an easy and safe cell-based assay suitable for studying the binding between SARS-CoV S protein and its receptor.
Figures

Similar articles
-
Amino acids 270 to 510 of the severe acute respiratory syndrome coronavirus spike protein are required for interaction with receptor.J Virol. 2004 May;78(9):4552-60. doi: 10.1128/jvi.78.9.4552-4560.2004. J Virol. 2004. PMID: 15078936 Free PMC article.
-
A study on antigenicity and receptor-binding ability of fragment 450-650 of the spike protein of SARS coronavirus.Virology. 2007 Mar 15;359(2):362-70. doi: 10.1016/j.virol.2006.09.022. Epub 2006 Oct 20. Virology. 2007. PMID: 17055551 Free PMC article.
-
Antigenicity and receptor-binding ability of recombinant SARS coronavirus spike protein.Biochem Biophys Res Commun. 2004 Jan 23;313(4):938-47. doi: 10.1016/j.bbrc.2003.11.180. Biochem Biophys Res Commun. 2004. PMID: 14706633 Free PMC article.
-
Cellular entry of the SARS coronavirus.Trends Microbiol. 2004 Oct;12(10):466-72. doi: 10.1016/j.tim.2004.08.008. Trends Microbiol. 2004. PMID: 15381196 Free PMC article. Review.
-
Expression, glycosylation, and modification of the spike (S) glycoprotein of SARS CoV.Methods Mol Biol. 2007;379:127-35. doi: 10.1007/978-1-59745-393-6_9. Methods Mol Biol. 2007. PMID: 17502675 Free PMC article. Review.
Cited by
-
Characterization of viral proteins encoded by the SARS-coronavirus genome.Antiviral Res. 2005 Feb;65(2):69-78. doi: 10.1016/j.antiviral.2004.10.001. Antiviral Res. 2005. PMID: 15708633 Free PMC article. Review.
-
Induction of specific immune responses by severe acute respiratory syndrome coronavirus spike DNA vaccine with or without interleukin-2 immunization using different vaccination routes in mice.Clin Vaccine Immunol. 2007 Jul;14(7):894-901. doi: 10.1128/CVI.00019-07. Epub 2007 May 9. Clin Vaccine Immunol. 2007. PMID: 17494640 Free PMC article.
-
The severe acute respiratory syndrome coronavirus 3a is a novel structural protein.Biochem Biophys Res Commun. 2005 Apr 29;330(1):286-92. doi: 10.1016/j.bbrc.2005.02.153. Biochem Biophys Res Commun. 2005. PMID: 15781262 Free PMC article.
-
ACE2 orthologues in non-mammalian vertebrates (Danio, Gallus, Fugu, Tetraodon and Xenopus).Gene. 2006 Aug 1;377:46-55. doi: 10.1016/j.gene.2006.03.010. Epub 2006 Apr 5. Gene. 2006. PMID: 16781089 Free PMC article.
-
Genetic immunization with Hantavirus vaccine combining expression of G2 glycoprotein and fused interleukin-2.Genet Vaccines Ther. 2008 Oct 22;6:15. doi: 10.1186/1479-0556-6-15. Genet Vaccines Ther. 2008. PMID: 18940009 Free PMC article.
References
-
- Chou C.F., Omary M.B. Mitotic arrest with anti-microtubule agents or okadaic acid is associated with increased glycoprotein terminal GlcNAc's. J. Cell Sci. 1994;107:1833–1843. - PubMed
-
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., Donovan M., Woolf B., Robison K., Jeyaseelan R., Breitbart R.E., Acton S. A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1–9. Circ. Res. 2000;87:E1–E9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

