High glucose is necessary for complete maturation of Pdx1-VP16-expressing hepatic cells into functional insulin-producing cells
- PMID: 15561947
- PMCID: PMC3422215
- DOI: 10.2337/diabetes.53.12.3168
High glucose is necessary for complete maturation of Pdx1-VP16-expressing hepatic cells into functional insulin-producing cells
Abstract
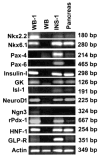
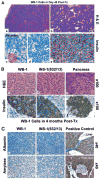
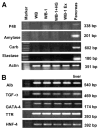
Pdx1 has been shown to convert hepatocytes into both exocrine and endocrine pancreatic cells in mice, but it fails to selectively convert hepatocytes into pure insulin-producing cells (IPCs). The molecular mechanisms underlying the transdifferentiation remain unclear. In this study, we generated a stably transfected rat hepatic cell line named WB-1 that expresses an active form of Pdx1 along with a reporter gene, RIP-eGFP. Our results demonstrate that Pdx1 induces the expression of multiple genes related to endocrine pancreas development and islet function in these liver cells. We do not however find any expression of the late-stage genes (Pax4, Pax6, Isl-1, and MafA) related to beta-cell development, and the cells do not secrete insulin upon the glucose challenge. Yet when WB-1 cells are transplanted into diabetic NOD-scid mice, these genes become activated and hyperglycemia is completely reversed. Detailed comparison of gene expression profiles between pre- and posttransplanted WB-1 cells demonstrates that the WB-1 cells have similar properties as that seen in pancreatic beta-cells. In addition, in vitro culture in high-glucose medium is sufficient to induce complete maturation of WB-1 cells into functional IPCs. In summary, we find that Pdx1-VP16 is able to selectively convert hepatic cells into pancreatic endocrine precursor cells. However, complete transdifferentiation into functional IPCs requires additional external factors, including high glucose or hyperglycemia. Thus, transdifferentiation of hepatocytes into functional IPCs may serve as a viable therapeutic option for patients with type 1 diabetes.
Figures

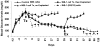


Similar articles
-
Liver stem cell-derived beta-cell surrogates for treatment of type 1 diabetes.Autoimmun Rev. 2006 Jul;5(6):409-13. doi: 10.1016/j.autrev.2005.10.009. Epub 2005 Dec 22. Autoimmun Rev. 2006. PMID: 16890895 Free PMC article. Review.
-
Role of Pax4 in Pdx1-VP16-mediated liver-to-endocrine pancreas transdifferentiation.Lab Invest. 2006 Aug;86(8):829-41. doi: 10.1038/labinvest.3700434. Epub 2006 May 29. Lab Invest. 2006. PMID: 16732298
-
Reprogramming liver-stem WB cells into functional insulin-producing cells by persistent expression of Pdx1- and Pdx1-VP16 mediated by lentiviral vectors.Lab Invest. 2006 Jan;86(1):83-93. doi: 10.1038/labinvest.3700368. Lab Invest. 2006. PMID: 16294197 Free PMC article.
-
Adult rat liver cells transdifferentiated with lentiviral IPF1 vectors reverse diabetes in mice: an ex vivo gene therapy approach.Diabetologia. 2007 Jan;50(1):121-30. doi: 10.1007/s00125-006-0509-8. Epub 2006 Nov 28. Diabetologia. 2007. PMID: 17131142
-
PDX1, Neurogenin-3, and MAFA: critical transcription regulators for beta cell development and regeneration.Stem Cell Res Ther. 2017 Nov 2;8(1):240. doi: 10.1186/s13287-017-0694-z. Stem Cell Res Ther. 2017. PMID: 29096722 Free PMC article. Review.
Cited by
-
Genetically reprogrammed, liver-derived insulin-producing cells are glucose-responsive, but susceptible to autoimmune destruction in settings of murine model of type 1 diabetes.Am J Transl Res. 2013;5(2):184-99. Epub 2013 Mar 28. Am J Transl Res. 2013. PMID: 23573363 Free PMC article.
-
Cathepsin B contributes to autophagy-related 7 (Atg7)-induced nod-like receptor 3 (NLRP3)-dependent proinflammatory response and aggravates lipotoxicity in rat insulinoma cell line.J Biol Chem. 2013 Oct 18;288(42):30094-30104. doi: 10.1074/jbc.M113.494286. Epub 2013 Aug 28. J Biol Chem. 2013. PMID: 23986436 Free PMC article.
-
In vitro generation of functional insulin-producing cells from human bone marrow-derived stem cells, but long-term culture running risk of malignant transformation.Am J Stem Cells. 2012 Jun 30;1(2):114-127. Epub 2012 Apr 28. Am J Stem Cells. 2012. PMID: 22833839 Free PMC article.
-
Liver stem cell-derived beta-cell surrogates for treatment of type 1 diabetes.Autoimmun Rev. 2006 Jul;5(6):409-13. doi: 10.1016/j.autrev.2005.10.009. Epub 2005 Dec 22. Autoimmun Rev. 2006. PMID: 16890895 Free PMC article. Review.
-
Implantation of bFGF-treated islet progenitor cells ameliorates streptozotocin-induced diabetes in rats.Acta Pharmacol Sin. 2010 Nov;31(11):1454-63. doi: 10.1038/aps.2010.130. Epub 2010 Oct 18. Acta Pharmacol Sin. 2010. PMID: 20953209 Free PMC article.
References
-
- Deutsch G, Jung J, Zheng M, Lora J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871– 881. - PubMed
-
- Rao MS, Reddy JK. Hepatic transdifferentiation in the pancreas. Semin Cell Biol. 1995;6:151–156. - PubMed
-
- Ferber S, Halkin A, Cohen H, Ber I, Einav Y, Goldberg I, Barshack I, Seijffers R, Kopolovic J, Kaiser N, Karasik A. Pancreatic and duodenal homeobox gene 1 induces expression of insulin genes in liver and ameliorates streptozotocin-induced hyperglycemia. Nat Med. 2000;6:568–572. - PubMed
-
- Horb ME, Shen CN, Tosh D, Slack JM. Experimental conversion of liver to pancreas. Curr Biol. 2003;13:105–115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

