The FG-repeat asymmetry of the nuclear pore complex is dispensable for bulk nucleocytoplasmic transport in vivo
- PMID: 15557115
- PMCID: PMC2172579
- DOI: 10.1083/jcb.200407156
The FG-repeat asymmetry of the nuclear pore complex is dispensable for bulk nucleocytoplasmic transport in vivo
Abstract
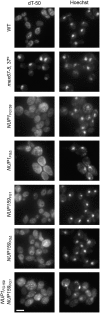
Nucleocytoplasmic transport occurs through gigantic proteinaceous channels called nuclear pore complexes (NPCs). Translocation through the NPC is exquisitely selective and is mediated by interactions between soluble transport carriers and insoluble NPC proteins that contain phenylalanine-glycine (FG) repeats. Although most FG nucleoporins (Nups) are organized symmetrically about the planar axis of the nuclear envelope, very few localize exclusively to one side of the NPC. We constructed Saccharomyces cerevisiae mutants with asymmetric FG repeats either deleted or swapped to generate NPCs with inverted FG asymmetry. The mutant Nups localize properly within the NPC and exhibit exchanged binding specificity for the export factor Xpo1. Surprisingly, we were unable to detect any defects in the Kap95, Kap121, Xpo1, or mRNA transport pathways in cells expressing the mutant FG Nups. These findings suggest that the biased distribution of FG repeats is not required for major nucleocytoplasmic trafficking events across the NPC.
Figures
Similar articles
-
Minimal nuclear pore complexes define FG repeat domains essential for transport.Nat Cell Biol. 2004 Mar;6(3):197-206. doi: 10.1038/ncb1097. Epub 2004 Feb 22. Nat Cell Biol. 2004. PMID: 15039779
-
Nuclear mRNA export requires specific FG nucleoporins for translocation through the nuclear pore complex.J Cell Biol. 2007 Sep 24;178(7):1121-32. doi: 10.1083/jcb.200704174. Epub 2007 Sep 17. J Cell Biol. 2007. PMID: 17875746 Free PMC article.
-
Nucleoporin's Like Charge Regions Are Major Regulators of FG Coverage and Dynamics Inside the Nuclear Pore Complex.PLoS One. 2015 Dec 11;10(12):e0143745. doi: 10.1371/journal.pone.0143745. eCollection 2015. PLoS One. 2015. PMID: 26658558 Free PMC article.
-
Structural biology of nucleocytoplasmic transport.Annu Rev Biochem. 2007;76:647-71. doi: 10.1146/annurev.biochem.76.052705.161529. Annu Rev Biochem. 2007. PMID: 17506639 Review.
-
Translocation through the nuclear pore complex: selectivity and speed by reduction-of-dimensionality.Traffic. 2005 May;6(5):421-7. doi: 10.1111/j.1600-0854.2005.00287.x. Traffic. 2005. PMID: 15813752 Review.
Cited by
-
Cargo surface hydrophobicity is sufficient to overcome the nuclear pore complex selectivity barrier.EMBO J. 2009 Sep 16;28(18):2697-705. doi: 10.1038/emboj.2009.225. Epub 2009 Aug 13. EMBO J. 2009. PMID: 19680225 Free PMC article.
-
Nup153 affects entry of messenger and ribosomal ribonucleoproteins into the nuclear basket during export.Mol Biol Cell. 2005 Dec;16(12):5610-20. doi: 10.1091/mbc.e05-08-0715. Epub 2005 Sep 29. Mol Biol Cell. 2005. PMID: 16195343 Free PMC article.
-
Design principles of selective transport through biopolymer barriers.Phys Rev E. 2019 Oct;100(4-1):042414. doi: 10.1103/PhysRevE.100.042414. Phys Rev E. 2019. PMID: 31770897 Free PMC article.
-
Nup50/Npap60 function in nuclear protein import complex disassembly and importin recycling.EMBO J. 2005 Nov 2;24(21):3681-9. doi: 10.1038/sj.emboj.7600843. Epub 2005 Oct 13. EMBO J. 2005. PMID: 16222336 Free PMC article.
-
Flexible gates: dynamic topologies and functions for FG nucleoporins in nucleocytoplasmic transport.Eukaryot Cell. 2009 Dec;8(12):1814-27. doi: 10.1128/EC.00225-09. Epub 2009 Oct 2. Eukaryot Cell. 2009. PMID: 19801417 Free PMC article. Review.
References
-
- Allen, N.P., L. Huang, A. Burlingame, and M. Rexach. 2001. Proteomic analysis of nucleoporin interacting proteins. J. Biol. Chem. 276:29268–29274. - PubMed
-
- Allen, N.P., S.S. Patel, L. Huang, R.J. Chalkley, A. Burlingame, M. Lutzmann, E.C. Hurt, and M. Rexach. 2002. Deciphering networks of protein interactions at the nuclear pore complex. Mol. Cell. Proteomics. 1:930–946. - PubMed
-
- Bayliss, R., T. Littlewood, and M. Stewart. 2000. Structural basis for the interaction between FxFG nucleoporin repeats and importin-beta in nuclear trafficking. Cell. 102:99–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

