In vivo effects of interleukin-17 on haematopoietic cells and cytokine release in normal mice
- PMID: 15548173
- PMCID: PMC6496772
- DOI: 10.1111/j.1365-2184.2004.00322.x
In vivo effects of interleukin-17 on haematopoietic cells and cytokine release in normal mice
Abstract
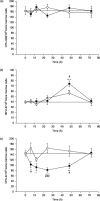
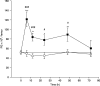
In order to gain more insight into mechanisms operating on the haematopoietic activity of the T-cell-derived cytokine, interleukin-17 (IL-17) and target cells that first respond to its action in vivo, the influence of a single intravenous injection of recombinant mouse IL-17 on bone marrow progenitors, further morphologically recognizable cells and peripheral blood cells was assessed in normal mice up to 72 h after treatment. Simultaneously, the release of IL-6, IL-10, IGF-I, IFN-gamma and NO by bone marrow cells was determined. Results showed that, in bone marrow, IL-17 did not affect granulocyte-macrophage (CFU-GM) progenitors, but induced a persistant increase in the number of morphologically recognizable proliferative granulocytes (PG) up to 48 h after treatment. The number of immature erythroid (BFU-E) progenitors was increased at 48 h, while the number of mature erythroid (CFU-E) progenitors was decreased up to 48 h. In peripheral blood, white blood cells were increased 6 h after treatment, mainly because of the increase in the number of lymphocytes. IL-17 also increased IL-6 release and NO production 6 h after administration. Additional in vitro assessment on bone marrow highly enriched Lin- progenitor cells, demonstrated a slightly enhancing effect of IL-17 on CFU-GM and no influence on BFU-E, suggesting the importance of bone marrow accessory cells and secondary induced cytokines for IL-17 mediated effects on progenitor cells. Taken together, these results demonstrate that in vivo IL-17 affects both granulocytic and erythroid lineages, with more mature haematopoietic progenitors responding first to its action. The opposite effects exerted on PG and CFU-E found at the same time indicate that IL-17, as a component of a regulatory network, is able to intervene in mechanisms that shift haematopoiesis from the erythroid to the granulocytic lineage.
Figures

Similar articles
-
The potential of interleukin-17 to mediate hematopoietic response.Immunol Res. 2012 Apr;52(1-2):34-41. doi: 10.1007/s12026-012-8276-8. Immunol Res. 2012. PMID: 22392050 Review.
-
Effect of IL-17 on in vitro hematopoietic progenitor cells growth and cytokine release in normal and post-irradiated murine bone marrow.Growth Factors. 2001;19(1):61-71. doi: 10.3109/08977190109001076. Growth Factors. 2001. PMID: 11678210
-
Combined effect of IL-17 and blockade of nitric oxide biosynthesis on haematopoiesis in mice.Acta Physiol (Oxf). 2010 May;199(1):31-41. doi: 10.1111/j.1748-1716.2010.02082.x. Epub 2010 Jan 22. Acta Physiol (Oxf). 2010. PMID: 20102341
-
The effect of interleukin-17 on hematopoietic cells and cytokine release in mouse spleen.Physiol Res. 2007;56(3):331-339. doi: 10.33549/physiolres.930944. Epub 2006 Jun 22. Physiol Res. 2007. PMID: 16792476
-
Effect of interleukin-1, GM-CSF, erythropoietin, and lithium on the toxicity associated with 3'-azido-3'-deoxythymidine (AZT) in vitro on hematopoietic progenitors (CFU-GM, CFU-MEG, and BFU-E) using murine retrovirus-infected hematopoietic cells.J Leukoc Biol. 1991 Dec;50(6):580-6. doi: 10.1002/jlb.50.6.580. J Leukoc Biol. 1991. PMID: 1940611
Cited by
-
Cytomics - importance of multimodal analysis of cell function and proliferation in oncology.Cell Prolif. 2006 Dec;39(6):495-505. doi: 10.1111/j.1365-2184.2006.00407.x. Cell Prolif. 2006. PMID: 17109634 Free PMC article. Review.
-
In vivo gene delivery with L-tyrosine polyphosphate nanoparticles.Mol Pharm. 2013 May 6;10(5):1836-44. doi: 10.1021/mp300623a. Epub 2013 Apr 8. Mol Pharm. 2013. PMID: 23510151 Free PMC article.
-
The potential of interleukin-17 to mediate hematopoietic response.Immunol Res. 2012 Apr;52(1-2):34-41. doi: 10.1007/s12026-012-8276-8. Immunol Res. 2012. PMID: 22392050 Review.
-
Interleukin-17 and its implication in the regulation of differentiation and function of hematopoietic and mesenchymal stem cells.Mediators Inflamm. 2015;2015:470458. doi: 10.1155/2015/470458. Epub 2015 Apr 27. Mediators Inflamm. 2015. PMID: 25999667 Free PMC article. Review.
-
Haplotype of non-synonymous mutations within IL-23R is associated with susceptibility to severe malaria anemia in a P. falciparum holoendemic transmission area of Kenya.BMC Infect Dis. 2017 Apr 20;17(1):291. doi: 10.1186/s12879-017-2404-y. BMC Infect Dis. 2017. PMID: 28427357 Free PMC article.
References
-
- Bugarski D, Jovčić G, Kataranovski M, Ivanović Z, Petakov M, Stojanović N, Milenković P (2000) Effects of treatment with interleukin‐1 receptor antagonist on endogenous interleukin‐1 levels in normal and irradiated mice. Physiol. Res. 49, 355. - PubMed
-
- Bugarski D, Krstić A, Vlaški M, Petakov M, Jovčić G, Stojanović N, Milenković P (2004) IL‐17 induced inhibitory effect on late stage murine erythroid bone marrow progenitors. Eur. Cytokine Netw. 15, 247. - PubMed
-
- Fossiez F, Djossou O, Chomarat P, Flores‐Romo L, Ait‐Yahia S, Maat C, Pin J, Garcia E, Saeland S, Blanchard D, Galliard C, Das Mahapatra B, Rouvier E, Golstein P, Banchereau J, Lebecque S (1996) T cell interleukin‐17 induces stromal cell to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 183, 2593. - PMC - PubMed
-
- Fossiez F, Banchereau J, Murray R, Van Kooten C, Garrone P, Lebecgue S (1998) Interleukin‐17. Int. Rev. Immunol. 16, 541. - PubMed
-
- Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok SJ, Tannenbaum SR (1982) Analysis of nitrate, nitrite and (15N) nitrite in biological fluids. Annal. Biochem. 126, 131. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

