Protein ubiquitination in postsynaptic densities after transient cerebral ischemia
- PMID: 15545915
- PMCID: PMC3518068
- DOI: 10.1097/01.WCB.0000136706.77918.21
Protein ubiquitination in postsynaptic densities after transient cerebral ischemia
Abstract
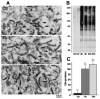
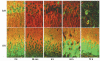
The mechanisms underlying neurologic deficits and delayed neuronal death after ischemia are not fully understood. In the present study, we report that transient cerebral ischemia induces accumulation of ubiquitinated proteins (ubi-proteins) in postsynaptic densities (PSDs). By immunoelectron microscopy, we demonstrated that ubi-proteins were highly accumulated in PSD structures after ischemia. On Western blots, ubi-proteins were markedly increased in purified PSDs at 30 minutes of reperfusion, and the increase persisted until cell death in the CA1 region after ischemia. In the resistant DG area, however, the changes were transient and significantly less pronounced. Deposition of ubi-proteins in PSDs after ischemia correlates well with PSD structural damage in the CA1 region as viewed by electron microscopy. These results suggest that the ubiquitin-proteasome system fails to repair and remove damaged proteins in PSDs. The changes may demolish synaptic neurotransmission, contribute to neurologic deficits, and eventually lead to delayed neuronal death after transient cerebral ischemia.
Figures

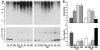
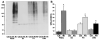
Similar articles
-
Protein aggregation after focal brain ischemia and reperfusion.J Cereb Blood Flow Metab. 2001 Jul;21(7):865-75. doi: 10.1097/00004647-200107000-00012. J Cereb Blood Flow Metab. 2001. PMID: 11435799
-
Modification of postsynaptic densities after transient cerebral ischemia: a quantitative and three-dimensional ultrastructural study.J Neurosci. 1999 Mar 15;19(6):1988-97. doi: 10.1523/JNEUROSCI.19-06-01988.1999. J Neurosci. 1999. PMID: 10066252 Free PMC article.
-
Transient cerebral ischemia increases tyrosine phosphorylation of the synaptic RAS-GTPase activating protein, SynGAP.J Cereb Blood Flow Metab. 2001 Aug;21(8):955-63. doi: 10.1097/00004647-200108000-00008. J Cereb Blood Flow Metab. 2001. PMID: 11487731
-
Alterations of hippocampal postsynaptic densities following transient ischemia.Hippocampus. 2000;10(5):610-6. doi: 10.1002/1098-1063(2000)10:5<610::AID-HIPO12>3.0.CO;2-E. Hippocampus. 2000. PMID: 11075832 Review.
-
Role of the ubiquitin-proteasome system in brain ischemia: friend or foe?Prog Neurobiol. 2014 Jan;112:50-69. doi: 10.1016/j.pneurobio.2013.10.003. Epub 2013 Oct 22. Prog Neurobiol. 2014. PMID: 24157661 Review.
Cited by
-
Role of the proteasome in excitotoxicity-induced cleavage of glutamic acid decarboxylase in cultured hippocampal neurons.PLoS One. 2010 Apr 12;5(4):e10139. doi: 10.1371/journal.pone.0010139. PLoS One. 2010. PMID: 20405034 Free PMC article.
-
Killer proteases and little strokes--how the things that do not kill you make you stronger.J Cereb Blood Flow Metab. 2007 Apr;27(4):655-68. doi: 10.1038/sj.jcbfm.9600380. Epub 2006 Aug 9. J Cereb Blood Flow Metab. 2007. PMID: 16896349 Free PMC article. Review.
-
Subcellular stress response and induction of molecular chaperones and folding proteins after transient global ischemia in rats.Brain Res. 2009 Jan 16;1249:9-18. doi: 10.1016/j.brainres.2008.10.032. Epub 2008 Oct 28. Brain Res. 2009. PMID: 18996359 Free PMC article.
-
Protein aggregation and proteasome dysfunction after brain ischemia.Stroke. 2007 Dec;38(12):3230-6. doi: 10.1161/STROKEAHA.107.487108. Epub 2007 Nov 1. Stroke. 2007. PMID: 17975104 Free PMC article.
-
Irreversible translation arrest in the reperfused brain.J Cereb Blood Flow Metab. 2007 May;27(5):875-93. doi: 10.1038/sj.jcbfm.9600388. Epub 2006 Aug 16. J Cereb Blood Flow Metab. 2007. PMID: 16926841 Free PMC article. Review.
References
-
- Asai A, Tanahashi N, Qiu JH, Saito N, Chi S, Kawahara N, Tanaka K, Kirino T. Selective proteasomal dysfunction in the hippocampal CA1 region after transient forebrain ischemia. J Cereb Blood Flow Metab. 2002;22:705–710. - PubMed
-
- Bloom FE, Aghajanian GK. Cytochemistry of synapses: Selective staining for electron microscopy. Science. 1968;154:1575–1577. - PubMed
-
- Burbea M, Dreier L, Dittman JS, Grunwald ME, Kaplan JM. Ubiquitin and AP180 regulate the abundance of GLR-1 glutamate receptors at postsynaptic elements in C elegans. Neuron. 2002;35:107–120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

