Inhibition of tumor necrosis factor (TNF) signal transduction by the adenovirus group C RID complex involves downregulation of surface levels of TNF receptor 1
- PMID: 15542663
- PMCID: PMC525002
- DOI: 10.1128/JVI.78.23.13113-13121.2004
Inhibition of tumor necrosis factor (TNF) signal transduction by the adenovirus group C RID complex involves downregulation of surface levels of TNF receptor 1
Abstract
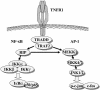
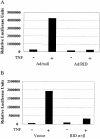
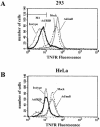
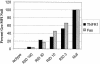
Adenoviruses employ multiple genes to inhibit the host antiviral responses. There is increasing evidence that these immunoregulatory genes may function either during lytic or latent infection. Adenovirus early transcription region 3 (E3) encodes at least seven proteins, five of which block the acquired or innate immune response. Previous findings from this laboratory demonstrated that the E3 proteins 10.4K and 14.5K, which form a complex in the plasma membrane, inhibit tumor necrosis factor (TNF)-induced activation of NF-kappaB and the synthesis of chemokines. To determine the mechanism of inhibition of these pathways by the adenovirus E3 10.4K/14.5K proteins, we have examined the effects of this viral complex on the inhibition of AP-1 and NF-kappaB activation by TNF and found a reduction in assembly of the TNF receptor 1 (TNFR1) signaling complex at the plasma membrane accompanied by downregulation of surface levels of TNFR1.
Figures




Similar articles
-
Mechanism for removal of tumor necrosis factor receptor 1 from the cell surface by the adenovirus RIDalpha/beta complex.J Virol. 2005 Nov;79(21):13606-17. doi: 10.1128/JVI.79.21.13606-13617.2005. J Virol. 2005. PMID: 16227281 Free PMC article.
-
Inhibition of tumor necrosis factor alpha-induced NF-kappa B activation by the adenovirus E3-10.4/14.5K complex.J Virol. 2002 Jun;76(11):5515-21. doi: 10.1128/jvi.76.11.5515-5521.2002. J Virol. 2002. PMID: 11991979 Free PMC article.
-
Adenovirus E3-10.4K/14.5K protein complex inhibits tumor necrosis factor-induced translocation of cytosolic phospholipase A2 to membranes.J Virol. 1997 Apr;71(4):2830-7. doi: 10.1128/JVI.71.4.2830-2837.1997. J Virol. 1997. PMID: 9060638 Free PMC article.
-
Adenovirus genes that modulate the sensitivity of virus-infected cells to lysis by TNF.J Cell Biochem. 1993 Dec;53(4):329-35. doi: 10.1002/jcb.240530410. J Cell Biochem. 1993. PMID: 8300750 Review.
-
Tumor necrosis factor alpha increases expression of adenovirus E3 proteins.Immunobiology. 1995 Jul;193(2-4):186-92. doi: 10.1016/s0171-2985(11)80542-5. Immunobiology. 1995. PMID: 8530142 Review.
Cited by
-
Regulation of the receptor for TNFalpha, TNFR1, in human conjunctival epithelial cells.Invest Ophthalmol Vis Sci. 2008 Sep;49(9):3992-8. doi: 10.1167/iovs.08-1873. Epub 2008 May 16. Invest Ophthalmol Vis Sci. 2008. PMID: 18487372 Free PMC article.
-
Ablation of the regulatory IE1 protein of murine cytomegalovirus alters in vivo pro-inflammatory TNF-alpha production during acute infection.PLoS Pathog. 2012;8(8):e1002901. doi: 10.1371/journal.ppat.1002901. Epub 2012 Aug 30. PLoS Pathog. 2012. PMID: 22952450 Free PMC article.
-
Adenovirus RIDalphabeta complex inhibits lipopolysaccharide signaling without altering TLR4 cell surface expression.J Virol. 2006 Jul;80(13):6378-86. doi: 10.1128/JVI.02350-05. J Virol. 2006. PMID: 16775326 Free PMC article.
-
The cytomegaloviral protein pUL138 acts as potentiator of tumor necrosis factor (TNF) receptor 1 surface density to enhance ULb'-encoded modulation of TNF-α signaling.J Virol. 2011 Dec;85(24):13260-70. doi: 10.1128/JVI.06005-11. Epub 2011 Oct 5. J Virol. 2011. PMID: 21976655 Free PMC article.
-
Modulation of tumor necrosis factor by microbial pathogens.PLoS Pathog. 2006 Feb;2(2):e4. doi: 10.1371/journal.ppat.0020004. PLoS Pathog. 2006. PMID: 16518473 Free PMC article. Review.
References
-
- Baud, V., and M. Karin. 2001. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 11:372-377. - PubMed
-
- Benedict, C. A., P. S. Norris, T. I. Prigozy, J. L. Bodmer, J. A. Mahr, C. T. Garnett, F. Martinon, J. Tschopp, L. R. Gooding, and C. F. Ware. 2001. Three adenovirus E3 proteins cooperate to evade apoptosis by tumor necrosis factor-related apoptosis-inducing ligand receptor-1 and -2. J. Biol. Chem. 276:3270-3278. - PubMed
-
- Bett, A. J., V. Krougliak, F. L. and Graha. 1995. DNA sequence of the deletion/insertion in early region 3 of Ad5 dl309. Virus Res. 39:75-82. - PubMed
-
- Carlin, C. R., A. E. Tollefson, H. A. Brady, B. L. Hoffman, and W. S. M. Wold. 1989. Epidermal growth factor receptor is down-regulated by a 10,400 mw protein encoded by the E3 region of adenovirus. Cell 57:135-144. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

