Somato-dendritic nicotinic receptor responses recorded in vitro from the medial septal diagonal band complex of the rodent
- PMID: 15528250
- PMCID: PMC1665480
- DOI: 10.1113/jphysiol.2004.070300
Somato-dendritic nicotinic receptor responses recorded in vitro from the medial septal diagonal band complex of the rodent
Abstract
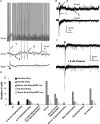
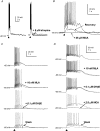
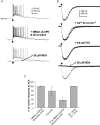
The medial septal diagonal band area (MS/DB), made up of GABAergic and cholinergic neurones, plays an essential role in the generation and modulation of the hippocampal theta rhythm. To understand the part that the cholinergic neurones might play in this activity, we sought to determine whether postsynaptic nicotinic receptor responses can be detected in slices of the rodent MS/DB by puffing on acetylcholine (ACh). Neurones were characterized electrophysiologically into GABAergic and cholinergic neurones according to previous criteria. Responses of the MS/SB neurones to ACh were various combinations of fast depolarizations (1.5-2.5 s), fast hyperpolarizations (3-4 s) and slow depolarizations (20-30 s), the latter two being blocked by atropine. The fast depolarizations were partially or not blocked with cadmium and low calcium, tetrodotoxin, and antagonists of other ionotropic receptors, and were antagonized with 25 microm mecamylamine. Pharmacological investigation of the responses showed that the alpha 7* nicotinic receptor type is associated with cholinergic neurones and 10% of the GABAergic neurones, and that non alpha 7* nicotinic receptor subtypes are associated with 50% of the GABAergic neurones. Pharmacological dissection of evoked and spontaneous postsynaptic responses, however, did not provide evidence for synaptic nicotinic receptor transmission in the MS/DB. It was concluded that nicotinic receptors, although prevalent on the somatic and/or dendritic membrane compartments of neurones in the MS/DB, are on extrasynaptic sites where they presumably play a neuromodulatory role. The presence of alpha 7* nicotinic receptors on cholinergic neurones may also render these cells specifically vulnerable to degeneration in Alzheimer's disease.
Figures

Similar articles
-
Septal innervation regulates the function of alpha7 nicotinic receptors in CA1 hippocampal interneurons.Exp Neurol. 2005 Oct;195(2):342-52. doi: 10.1016/j.expneurol.2005.05.006. Exp Neurol. 2005. PMID: 16000197
-
A functional glutamatergic neurone network in the medial septum and diagonal band area.J Physiol. 2005 Aug 1;566(Pt 3):865-84. doi: 10.1113/jphysiol.2005.089664. Epub 2005 May 26. J Physiol. 2005. PMID: 15919710 Free PMC article.
-
Fast synaptic transmission mediated by alpha-bungarotoxin-sensitive nicotinic acetylcholine receptors in lamina X neurones of neonatal rat spinal cord.J Physiol. 2002 Nov 1;544(3):727-39. doi: 10.1113/jphysiol.2002.028894. J Physiol. 2002. PMID: 12411519 Free PMC article.
-
Modulation of GABAergic synaptic transmission by terminal nicotinic acetylcholine receptors in the central autonomic nucleus of the neonatal rat spinal cord.Neuropharmacology. 2006 Jul;51(1):77-89. doi: 10.1016/j.neuropharm.2006.03.007. Epub 2006 May 5. Neuropharmacology. 2006. PMID: 16678861
-
GABAB receptors in the medial septum/diagonal band slice from 16-25 day rat.Neuroscience. 2005;132(3):789-800. doi: 10.1016/j.neuroscience.2005.01.027. Neuroscience. 2005. PMID: 15837139
Cited by
-
A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides.J Neurosci. 2009 Jan 28;29(4):918-29. doi: 10.1523/JNEUROSCI.3952-08.2009. J Neurosci. 2009. PMID: 19176801 Free PMC article.
-
Functional alpha7 nicotinic receptors are expressed on immature granule cells of the postnatal dentate gyrus.Brain Res. 2015 Mar 19;1601:15-30. doi: 10.1016/j.brainres.2014.12.041. Epub 2014 Dec 29. Brain Res. 2015. PMID: 25553616 Free PMC article.
-
Varenicline and nicotine enhance GABAergic synaptic transmission in rat CA1 hippocampal and medial septum/diagonal band neurons.Life Sci. 2013 Mar 14;92(6-7):337-44. doi: 10.1016/j.lfs.2012.12.013. Epub 2013 Jan 24. Life Sci. 2013. PMID: 23352971 Free PMC article.
-
Induction by kainate of theta frequency rhythmic activity in the rat medial septum-diagonal band complex in vitro.J Physiol. 2005 Apr 1;564(Pt 1):83-102. doi: 10.1113/jphysiol.2004.080622. Epub 2005 Jan 27. J Physiol. 2005. PMID: 15677688 Free PMC article.
-
Nicotine induction of theta frequency oscillations in rodent medial septal diagonal band in vitro.Acta Pharmacol Sin. 2013 Jun;34(6):819-29. doi: 10.1038/aps.2012.198. Epub 2013 Mar 25. Acta Pharmacol Sin. 2013. PMID: 23524566 Free PMC article.
References
-
- Albuquerque EX, Alkondon M, Pereira EF, Castro NG, Schrattenholz A, Barbosa CT, Bonfante-Cabarcas R, Aracava Y, Eisenberg HM, Maelicke A. Properties of neuronal nicotinic acetylcholine receptors: pharmacological characterization and modulation of synaptic function. J Pharmacol Exp Ther. 1997;280:1117–1136. - PubMed
-
- Alkondon M, Pereira EF, Albuquerque EX. Alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res. 1998;810:257–263. 10.1016/S0006-8993(98)00880-4. - DOI - PubMed
-
- Alonso A, Gaztelu JM, Buno W, Jr, Garcia-Austt E. Cross-correlation analysis of septohippocampal neurons during theta-rhythm. Brain Res. 1987;413:135–146. 10.1016/0006-8993(87)90162-4. - DOI - PubMed
-
- Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal formation of the rat. J Comp Neurol. 1985;240:37–59. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

