Cell-density-dependent regulation of neural precursor cell function
- PMID: 15522966
- PMCID: PMC528770
- DOI: 10.1073/pnas.0407065101
Cell-density-dependent regulation of neural precursor cell function
Abstract
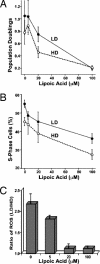
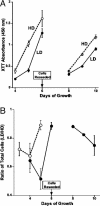
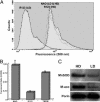
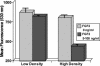
Stress-induced reductions of neural precursor cells from the subgranular zone of the hippocampal dentate gyrus have been linked to impaired neurogenesis and cognitive dysfunction. Given the importance of redox state in regulating multiple damage-responsive pathways in the CNS, we hypothesize that oxidative stress plays a major role in affecting neurogenesis and subsequent cognitive function after cell injury/depletion. Using an in vitro system, we showed that the level of reactive oxygen species (ROS), which depend critically on changes in cell density, were significantly higher in neural precursor cells when compared with other primary and transformed cell lines. ROS were significantly elevated ( approximately 4-fold) under low- (<1 x 10(4) cells per cm(2)) versus high-density (>1 x 10(5) cells per cm(2)) conditions. Higher ROS levels found at lower cell densities were associated with elevated proliferation and increased metabolic activity. These ROS were likely a result of altered mitochondrial function that ultimately compromised the growth rate of cells. At high cell densities, intracellular ROS and oxidative damage were reduced in concert with an increased expression of mitochondrial superoxide dismutase 2. Our finding that DNA-damage-induced depletion of neural precursor cells in the subgranular zone of mice also led to increased ROS and altered proliferation validated our in vitro system. Increased ROS and proliferation associated with the reduction of precursor cell numbers both in vitro and in vivo could be reversed with the antioxidant alpha-lipoic acid. These data showed that neural precursor cells were predisposed to microenvironmental cues that regulate redox-sensitive pathways to control cellular proliferation after CNS damage.
Figures

Similar articles
-
Using superoxide dismutase/catalase mimetics to manipulate the redox environment of neural precursor cells.Radiat Prot Dosimetry. 2006;122(1-4):228-36. doi: 10.1093/rpd/ncl458. Epub 2006 Dec 13. Radiat Prot Dosimetry. 2006. PMID: 17166877
-
Radiation response of neural precursor cells: linking cellular sensitivity to cell cycle checkpoints, apoptosis and oxidative stress.Radiat Res. 2004 Jan;161(1):17-27. doi: 10.1667/rr3112. Radiat Res. 2004. PMID: 14680400
-
Efficient production of reactive oxygen species in neural precursor cells after exposure to 250 MeV protons.Radiat Res. 2005 Oct;164(4 Pt 2):540-4. doi: 10.1667/rr3369.1. Radiat Res. 2005. PMID: 16187784
-
Mitochondrial and Autophagic Regulation of Adult Neurogenesis in the Healthy and Diseased Brain.Int J Mol Sci. 2021 Mar 24;22(7):3342. doi: 10.3390/ijms22073342. Int J Mol Sci. 2021. PMID: 33805219 Free PMC article. Review.
-
Free radicals, metals and antioxidants in oxidative stress-induced cancer.Chem Biol Interact. 2006 Mar 10;160(1):1-40. doi: 10.1016/j.cbi.2005.12.009. Epub 2006 Jan 23. Chem Biol Interact. 2006. PMID: 16430879 Review.
Cited by
-
Genomic stability of mouse spermatogonial stem cells in vitro.Sci Rep. 2021 Dec 17;11(1):24199. doi: 10.1038/s41598-021-03658-1. Sci Rep. 2021. PMID: 34921203 Free PMC article.
-
Oxidative stress and gamma radiation-induced cancellous bone loss with musculoskeletal disuse.J Appl Physiol (1985). 2010 Jan;108(1):152-61. doi: 10.1152/japplphysiol.00294.2009. Epub 2009 Oct 29. J Appl Physiol (1985). 2010. PMID: 19875718 Free PMC article.
-
Retrovirus-induced oxidative stress with neuroimmunodegeneration is suppressed by antioxidant treatment with a refined monosodium alpha-luminol (Galavit).J Virol. 2006 May;80(9):4557-69. doi: 10.1128/JVI.80.9.4557-4569.2006. J Virol. 2006. PMID: 16611916 Free PMC article.
-
Potential Therapeutic Effects of Lipoic Acid on Memory Deficits Related to Aging and Neurodegeneration.Front Pharmacol. 2017 Dec 12;8:849. doi: 10.3389/fphar.2017.00849. eCollection 2017. Front Pharmacol. 2017. PMID: 29311912 Free PMC article. Review.
-
Efficient cultivation conditions for human limbal epithelial cells.J Korean Med Sci. 2008 Oct;23(5):864-9. doi: 10.3346/jkms.2008.23.5.864. J Korean Med Sci. 2008. PMID: 18955795 Free PMC article.
References
-
- Eriksson, P. S., Perfilieva, E., Bjork-Eriksson, T., Alborn, A. M., Nordborg, C., Peterson, D. A. & Gage, F. H. (1998) Nat. Med. 4, 1313–1317. - PubMed
-
- Raber, J., Rola, R., LeFevour, A., Morhardt, D., Curley, J., Mizumatsu, S. & Fike, J. (2004) Radiat. Res. 162, 39–47. - PubMed
-
- Rola, R., Raber, J., Rizk, A., Otsuka, S., VandenBerg, S., Morhardt, D. & Fike, J. (2004) Exp. Neurol. 188, 316–330. - PubMed
-
- Monje, M. L., Mizumatsu, S., Fike, J. R. & Palmer, T. D. (2002) Nat. Med. 8, 955–962. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

