alpha-Synuclein produces a long-lasting increase in neurotransmitter release
- PMID: 15510220
- PMCID: PMC526467
- DOI: 10.1038/sj.emboj.7600451
alpha-Synuclein produces a long-lasting increase in neurotransmitter release
Abstract
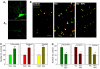
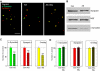
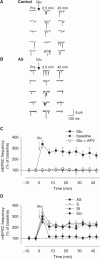
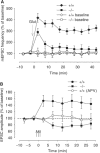
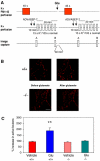
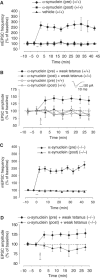
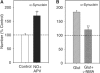
Wild-type alpha-synuclein, a protein of unknown function, has received much attention because of its involvement in a series of diseases that are known as synucleinopathies. We find that long-lasting potentiation of synaptic transmission between cultured hippocampal neurons is accompanied by an increase in the number of alpha-synuclein clusters. Conversely, suppression of alpha-synuclein expression through antisense nucleotide and knockout techniques blocks the potentiation, as well as the glutamate-induced increase in presynaptic functional bouton number. Consistent with these findings, alpha-synuclein introduction into the presynaptic neuron of a pair of monosynaptically connected cells causes a rapid and long-lasting enhancement of synaptic transmission, and rescues the block of potentiation in alpha-synuclein null mouse cultures. Also, we report that the application of nitric oxide (NO) increases the number of alpha-synuclein clusters, and inhibitors of NO-synthase block this increase, supporting the hypothesis that NO is involved in the enhancement of the number of alpha-synuclein clusters. Thus, alpha-synuclein is involved in synaptic plasticity by augmenting transmitter release from the presynaptic terminal.
Figures
Similar articles
-
Stressor-related impairment of synaptic transmission in hippocampal slices from alpha-synuclein knockout mice.Eur J Neurosci. 2004 Dec;20(11):3085-91. doi: 10.1111/j.1460-9568.2004.03801.x. Eur J Neurosci. 2004. PMID: 15579163
-
Alpha-synuclein involvement in hippocampal synaptic plasticity: role of NO, cGMP, cGK and CaMKII.Eur J Neurosci. 2007 Jun;25(12):3583-96. doi: 10.1111/j.1460-9568.2007.05569.x. Eur J Neurosci. 2007. PMID: 17610578
-
Double-knockout mice for alpha- and beta-synucleins: effect on synaptic functions.Proc Natl Acad Sci U S A. 2004 Oct 12;101(41):14966-71. doi: 10.1073/pnas.0406283101. Epub 2004 Oct 1. Proc Natl Acad Sci U S A. 2004. PMID: 15465911 Free PMC article.
-
Alpha-synuclein: between synaptic function and dysfunction.Histol Histopathol. 2003 Oct;18(4):1257-66. doi: 10.14670/HH-18.1257. Histol Histopathol. 2003. PMID: 12973692 Review.
-
Synucleins in synaptic plasticity and neurodegenerative disorders.J Neurosci Res. 1999 Oct 1;58(1):120-9. J Neurosci Res. 1999. PMID: 10491577 Review.
Cited by
-
Is Cell Death Primary or Secondary in the Pathophysiology of Idiopathic Parkinson's Disease?Biomolecules. 2015 Jul 16;5(3):1467-79. doi: 10.3390/biom5031467. Biomolecules. 2015. PMID: 26193328 Free PMC article. Review.
-
The Interplay between Alpha-Synuclein Clearance and Spreading.Biomolecules. 2015 Apr 14;5(2):435-71. doi: 10.3390/biom5020435. Biomolecules. 2015. PMID: 25874605 Free PMC article. Review.
-
Alpha-synuclein: from secretion to dysfunction and death.Cell Death Dis. 2012 Jul 19;3(7):e350. doi: 10.1038/cddis.2012.94. Cell Death Dis. 2012. PMID: 22825468 Free PMC article. Review.
-
Biophysical processes underlying cross-seeding in amyloid aggregation and implications in amyloid pathology.Biophys Chem. 2021 Feb;269:106507. doi: 10.1016/j.bpc.2020.106507. Epub 2020 Nov 19. Biophys Chem. 2021. PMID: 33254009 Free PMC article. Review.
-
From Synaptic Physiology to Synaptic Pathology: The Enigma of α-Synuclein.Int J Mol Sci. 2024 Jan 12;25(2):986. doi: 10.3390/ijms25020986. Int J Mol Sci. 2024. PMID: 38256059 Free PMC article. Review.
References
-
- Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A (2000) Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25: 239–252 - PubMed
-
- Antonova I, Arancio O, Trillat AC, Wang HG, Zablow L, Udo H, Kandel ER, Hawkins RD (2001) Rapid increase in immunoreactive presynaptic terminals during long-lasting potentiation. Science 294: 1547–1550 - PubMed
-
- Antonova I, Trillat AC, Arancio O, De Vente J, Hawkins RD (1999) Glutamate and nitric oxide produce increases in cGMP and synaptophysin immunofluorescence in cultured hippocampal neurons. Soc Neurosci Abstr 25: 733
-
- Arancio O, Kandel ER, Hawkins RD (1995) Activity-dependent long-term enhancement of transmitter release by presynaptic 3′, 5′ -cyclic GMP in cultured hippocampal neurons. Nature 376: 74–80 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

