E2Fs link the control of G1/S and G2/M transcription
- PMID: 15510213
- PMCID: PMC533046
- DOI: 10.1038/sj.emboj.7600459
E2Fs link the control of G1/S and G2/M transcription
Abstract
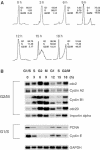
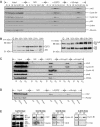
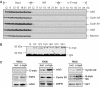
Previous work has provided evidence for E2F-dependent transcription control of both G1/S- and G2/M-regulated genes. Analysis of the G2-regulated cdc2 and cyclin B1 genes reveals the presence of both positive- and negative-acting E2F promoter elements. Additional elements provide both positive (CCAAT and Myb) and negative (CHR) control. Chromatin immunoprecipitation assays identify multiple interactions of E2F proteins that include those previously shown to activate and repress transcription. We find that E2F1, E2F2, and E2F3 bind to the positive-acting E2F site in the cdc2 promoter, whereas E2F4 binds to the negative-acting site. We also find that binding of an activator E2F is dependent on an adjacent CCAAT site that is bound by the NF-Y transcription factor and binding of a repressor E2F is dependent on an adjacent CHR element, suggesting a role for cooperative interactions in determining both activation and repression. Finally, the kinetics of B-Myb interaction with the G2-regulated promoters coincides with the activation of the genes, and RNAi-mediated reduction of B-Myb inhibits expression of cyclin B1 and cdc2. The ability of B-Myb to interact with the cdc2 promoter is dependent on an intact E2F binding site. These results thus point to a role for E2Fs, together with B-Myb, which is an E2F-regulated gene expressed at G1/S, in linking the regulation of genes at G1/S and G2/M.
Figures





Similar articles
-
Distinct recruitment of E2F family members to specific E2F-binding sites mediates activation and repression of the E2F1 promoter.Oncogene. 2003 Oct 23;22(48):7632-41. doi: 10.1038/sj.onc.1206840. Oncogene. 2003. PMID: 14576826
-
Correlation between E2F-1 requirement in the S phase and E2F-1 transactivation of cell cycle-related genes in human cells.Cancer Res. 1994 Mar 15;54(6):1402-6. Cancer Res. 1994. PMID: 8137237
-
E2F binding is required but not sufficient for repression of B-myb transcription in quiescent fibroblasts.Oncogene. 1996 Sep 5;13(5):1073-82. Oncogene. 1996. PMID: 8806697
-
Regulation of the G1/S transition phase in mesangial cells by E2F1.Kidney Int. 1999 Oct;56(4):1238-41. doi: 10.1046/j.1523-1755.1999.00705.x. Kidney Int. 1999. PMID: 10504464 Review.
-
A quantitative model for the cdc2 control of S phase and mitosis in fission yeast.Trends Genet. 1996 Sep;12(9):345-50. Trends Genet. 1996. PMID: 8855663 Review.
Cited by
-
Increased MYBL2 expression in aggressive hormone-sensitive prostate cancer.Mol Oncol. 2022 Dec;16(22):3994-4010. doi: 10.1002/1878-0261.13314. Epub 2022 Oct 2. Mol Oncol. 2022. PMID: 36087093 Free PMC article.
-
OGFOD1 is required for breast cancer cell proliferation and is associated with poor prognosis in breast cancer.Oncotarget. 2015 Aug 14;6(23):19528-41. doi: 10.18632/oncotarget.3683. Oncotarget. 2015. PMID: 25909288 Free PMC article.
-
Cancerous inhibitor of protein phosphatase 2A contributes to human papillomavirus oncoprotein E7-induced cell proliferation via E2F1.Oncotarget. 2015 Mar 10;6(7):5253-62. doi: 10.18632/oncotarget.2867. Oncotarget. 2015. PMID: 25650660 Free PMC article.
-
MYBL2, a link between proliferation and differentiation in maturing colon epithelial cells.J Cell Physiol. 2011 Mar;226(3):785-91. doi: 10.1002/jcp.22399. J Cell Physiol. 2011. PMID: 20857481 Free PMC article.
-
The human synMuv-like protein LIN-9 is required for transcription of G2/M genes and for entry into mitosis.EMBO J. 2007 Jan 10;26(1):144-57. doi: 10.1038/sj.emboj.7601478. Epub 2006 Dec 7. EMBO J. 2007. PMID: 17159899 Free PMC article.
References
-
- Araki K, Nakajima Y, Eto K, Ikeda MA (2003) Distinct recruitment of E2F family members to specific E2F-binding sites mediates activation and repression of the E2F1 promoter. Oncogene 22: 7632–7641 - PubMed
-
- DeGregori J, Leone G, Ohtani K, Miron A, Nevins JR (1995) E2F1 accumulation bypasses a G1 arrest resulting from the inhibition of G1 cyclin-dependent kinase activity. Genes Dev 9: 2873–2887 - PubMed
-
- Dyson N (1998) The regulation of E2F by pRB-family proteins. Genes Dev 12: 2245–2262 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

