Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice
- PMID: 15509788
- PMCID: PMC525490
- DOI: 10.1128/MCB.24.22.9848-9862.2004
Neuroprotective role of the Reaper-related serine protease HtrA2/Omi revealed by targeted deletion in mice
Abstract
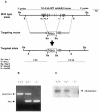
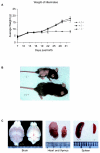
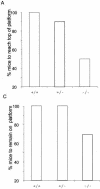
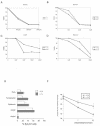
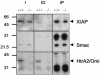
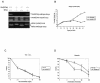
The serine protease HtrA2/Omi is released from the mitochondrial intermembrane space following apoptotic stimuli. Once in the cytosol, HtrA2/Omi has been implicated in promoting cell death by binding to inhibitor of apoptosis proteins (IAPs) via its amino-terminal Reaper-related motif, thus inducing caspase activity, and also in mediating caspase-independent death through its own protease activity. We report here the phenotype of mice entirely lacking expression of HtrA2/Omi due to targeted deletion of its gene, Prss25. These animals, or cells derived from them, show no evidence of reduced rates of cell death but on the contrary suffer loss of a population of neurons in the striatum, resulting in a neurodegenerative disorder with a parkinsonian phenotype that leads to death of the mice around 30 days after birth. The phenotype of these mice suggests that it is the protease function of this protein and not its IAP binding motif that is critical. This conclusion is reinforced by the finding that simultaneous deletion of the other major IAP binding protein, Smac/DIABLO, does not obviously alter the phenotype of HtrA2/Omi knockout mice or cells derived from them. Mammalian HtrA2/Omi is therefore likely to function in vivo in a manner similar to that of its bacterial homologues DegS and DegP, which are involved in protection against cell stress, and not like the proapoptotic Reaper family proteins in Drosophila melanogaster.
Figures




Similar articles
-
The serine protease Omi/HtrA2 regulates apoptosis by binding XIAP through a reaper-like motif.J Biol Chem. 2002 Jan 4;277(1):439-44. doi: 10.1074/jbc.M109784200. Epub 2001 Oct 15. J Biol Chem. 2002. PMID: 11602612
-
Binding specificity and regulation of the serine protease and PDZ domains of HtrA2/Omi.J Biol Chem. 2003 Dec 5;278(49):49417-27. doi: 10.1074/jbc.M308659200. Epub 2003 Sep 25. J Biol Chem. 2003. PMID: 14512424
-
Inactivation of Omi/HtrA2 protease leads to the deregulation of mitochondrial Mulan E3 ubiquitin ligase and increased mitophagy.Biochim Biophys Acta. 2014 Jul;1843(7):1295-307. doi: 10.1016/j.bbamcr.2014.03.027. Epub 2014 Apr 5. Biochim Biophys Acta. 2014. PMID: 24709290
-
The mitochondrial serine protease HtrA2/Omi: an overview.Cell Death Differ. 2008 Mar;15(3):453-60. doi: 10.1038/sj.cdd.4402291. Epub 2008 Jan 4. Cell Death Differ. 2008. PMID: 18174901 Review.
-
Mammalian mitochondrial IAP binding proteins.Biochem Biophys Res Commun. 2003 May 9;304(3):499-504. doi: 10.1016/s0006-291x(03)00622-3. Biochem Biophys Res Commun. 2003. PMID: 12729584 Review.
Cited by
-
The PARL family of mitochondrial rhomboid proteases.Semin Cell Dev Biol. 2010 Aug;21(6):582-92. doi: 10.1016/j.semcdb.2009.12.011. Epub 2010 Jan 4. Semin Cell Dev Biol. 2010. PMID: 20045481 Free PMC article. Review.
-
Different mitochondrial intermembrane space proteins are released during apoptosis in a manner that is coordinately initiated but can vary in duration.Proc Natl Acad Sci U S A. 2006 Aug 1;103(31):11573-8. doi: 10.1073/pnas.0603007103. Epub 2006 Jul 24. Proc Natl Acad Sci U S A. 2006. PMID: 16864784 Free PMC article.
-
Disease model organism for Parkinson disease: Drosophila melanogaster.BMB Rep. 2019 Apr;52(4):250-258. doi: 10.5483/BMBRep.2019.52.4.204. BMB Rep. 2019. PMID: 30545438 Free PMC article. Review.
-
Mitochondrial Chaperones in the Brain: Safeguarding Brain Health and Metabolism?Front Endocrinol (Lausanne). 2018 Apr 26;9:196. doi: 10.3389/fendo.2018.00196. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 29755410 Free PMC article.
-
Parkinson Disease from Mendelian Forms to Genetic Susceptibility: New Molecular Insights into the Neurodegeneration Process.Cell Mol Neurobiol. 2018 Aug;38(6):1153-1178. doi: 10.1007/s10571-018-0587-4. Epub 2018 Apr 26. Cell Mol Neurobiol. 2018. PMID: 29700661 Free PMC article. Review.
References
-
- Darzynkiewicz, Z., and E. Bedner. 2000. Analysis of apoptotic cells by flow and laser scanning cytometry. Methods Enzymol. 322:18-39. - PubMed
-
- Du, C., M. Fang, Y. Li, L. Li, and X. Wang. 2000. Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102:33-42. - PubMed
-
- Faccio, L., C. Fusco, A. Chen, S. Martinotti, J. V. Bonventre, and A. S. Zervos. 2000. Characterization of a novel human serine protease that has extensive homology to bacterial heat shock endoprotease HtrA and is regulated by kidney ischemia. J. Biol. Chem. 275:2581-2588. - PubMed
-
- Ghadially, F. N. 1982. Ultrastructural pathology of the cell and matrix, 2nd ed. Butterworths, London, United Kingdom.
-
- Gray, C. W., R. V. Ward, E. Karran, S. Turconi, A. Rowles, D. Viglienghi, C. Southan, A. Barton, K. G. Fantom, A. West, J. Savopoulos, N. J. Hassan, H. Clinkenbeard, C. Hanning, B. Amegadzie, J. B. Davis, C. Dingwall, G. P. Livi, and C. L. Creasy. 2000. Characterization of human HtrA2, a novel serine protease involved in the mammalian cellular stress response. Eur. J. Biochem. 267:5699-5710. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
