Mitochondrial proteomic analysis of a cell line model of familial amyotrophic lateral sclerosis
- PMID: 15501831
- PMCID: PMC1360176
- DOI: 10.1074/mcp.M400094-MCP200
Mitochondrial proteomic analysis of a cell line model of familial amyotrophic lateral sclerosis
Abstract
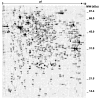
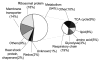
Mutations in copper-zinc superoxide dismutase (SOD1) have been linked to a subset of familial amytrophic lateral sclerosis (fALS), a fatal neurodegenerative disease characterized by progressive motor neuron death. An increasing amount of evidence supports that mitochondrial dysfunction and apoptosis activation play a critical role in the fALS etiology, but little is known about the mechanisms by which SOD1 mutants cause the mitochondrial dysfunction and apoptosis. In this study, we use proteomic approaches to identify the mitochondrial proteins that are altered in the presence of a fALS-causing mutant G93A-SOD1. A comprehensive characterization of mitochondrial proteins from NSC34 cells, a motor neuron-like cell line, was achieved by two independent proteomic approaches. Four hundred seventy unique proteins were identified in the mitochondrial fraction collectively, 75 of which are newly discovered proteins that previously had only been reported at the cDNA level. Two-dimensional gel electrophoresis was subsequently used to analyze the differences between the mitochondrial proteomes of NSC34 cells expressing wild-type and G93A-SOD1. Nine and 36 protein spots displayed elevated and suppressed abundance respectively in G93A-SOD1-expressing cells. The 45 spots were identified by MS, and they include proteins involved in mitochondrial membrane transport, apoptosis, the respiratory chain, and molecular chaperones. In particular, alterations in the post-translational modifications of voltage-dependent anion channel 2 (VDAC2) were found, and its relevance to regulating mitochondrial membrane permeability and activation of apoptotic pathways is discussed. The potential role of other proteins in the mutant SOD1-mediated fALS is also discussed. This study has produced a short list of mitochondrial proteins that may hold the key to the mechanisms by which SOD1 mutants cause mitochondrial dysfunction and neuronal death. It has laid the foundation for further detailed functional studies to elucidate the role of particular mitochondrial proteins, such as VDAC2, in the pathogenesis of familial ALS.
Figures
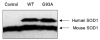


Similar articles
-
2-DE and MALDI-TOF-MS for a comparative analysis of proteins expressed in different cellular models of amyotrophic lateral sclerosis.Electrophoresis. 2007 Dec;28(23):4320-9. doi: 10.1002/elps.200700455. Electrophoresis. 2007. PMID: 17979159
-
Mitochondrial dysfunction in a cell culture model of familial amyotrophic lateral sclerosis.Brain. 2002 Jul;125(Pt 7):1522-33. doi: 10.1093/brain/awf167. Brain. 2002. PMID: 12077002
-
Palmitoylation of superoxide dismutase 1 (SOD1) is increased for familial amyotrophic lateral sclerosis-linked SOD1 mutants.J Biol Chem. 2013 Jul 26;288(30):21606-17. doi: 10.1074/jbc.M113.487231. Epub 2013 Jun 12. J Biol Chem. 2013. PMID: 23760509 Free PMC article.
-
Mitochondrial dysfunction and amyotrophic lateral sclerosis.Muscle Nerve. 2006 May;33(5):598-608. doi: 10.1002/mus.20489. Muscle Nerve. 2006. PMID: 16372325 Review.
-
SOD1 in neurotoxicity and its controversial roles in SOD1 mutation-negative ALS.Adv Biol Regul. 2016 Jan;60:95-104. doi: 10.1016/j.jbior.2015.10.006. Epub 2015 Oct 31. Adv Biol Regul. 2016. PMID: 26563614 Review.
Cited by
-
Mic13 Is Essential for Formation of Crista Junctions in Mammalian Cells.PLoS One. 2016 Aug 1;11(8):e0160258. doi: 10.1371/journal.pone.0160258. eCollection 2016. PLoS One. 2016. PMID: 27479602 Free PMC article.
-
Mitochondrial dynamism and the pathogenesis of Amyotrophic Lateral Sclerosis.Front Cell Neurosci. 2015 Feb 10;9:31. doi: 10.3389/fncel.2015.00031. eCollection 2015. Front Cell Neurosci. 2015. PMID: 25713513 Free PMC article. Review.
-
AK2 Promotes the Migration and Invasion of Lung Adenocarcinoma by Activating TGF-β/Smad Pathway In vitro and In vivo.Front Pharmacol. 2021 Sep 22;12:714365. doi: 10.3389/fphar.2021.714365. eCollection 2021. Front Pharmacol. 2021. PMID: 34630090 Free PMC article.
-
Mitochondrial dysfunction in SOD1G93A-bearing astrocytes promotes motor neuron degeneration: prevention by mitochondrial-targeted antioxidants.J Neurosci. 2008 Apr 16;28(16):4115-22. doi: 10.1523/JNEUROSCI.5308-07.2008. J Neurosci. 2008. PMID: 18417691 Free PMC article.
-
VDAC1 at the Intersection of Cell Metabolism, Apoptosis, and Diseases.Biomolecules. 2020 Oct 26;10(11):1485. doi: 10.3390/biom10111485. Biomolecules. 2020. PMID: 33114780 Free PMC article. Review.
References
-
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. - PubMed
-
- Deng HX, Hentati A, Tainer JA, Iqbal Z, Cayabyab A, Hung WY, Getzoff ED, Hu P, Herzfeldt B, Roos RP, et al. Amyotrophic lateral sclerosis and structural defects in Cu, Zn superoxide dismutase. Science. 1993;261:1047–1051. - PubMed
-
- Gaudette M, Hirano M, Siddique T. Current status of SOD1 mutations in familial amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord. 2000;1:83–89. - PubMed
-
- Brown RH., Jr SOD1 aggregates in ALS: Cause, correlate or consequence? Nat Med. 1998;4:1362–1364. - PubMed
-
- Cleveland DW, Liu J. Oxidation versus aggregation—How do SOD1 mutants cause ALS? Nat Med. 2000;6:1320–1321. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

