BRUCE, a giant E2/E3 ubiquitin ligase and inhibitor of apoptosis protein of the trans-Golgi network, is required for normal placenta development and mouse survival
- PMID: 15485903
- PMCID: PMC522247
- DOI: 10.1128/MCB.24.21.9339-9350.2004
BRUCE, a giant E2/E3 ubiquitin ligase and inhibitor of apoptosis protein of the trans-Golgi network, is required for normal placenta development and mouse survival
Abstract
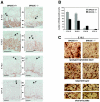
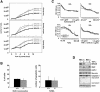
BRUCE is a highly conserved 528-kDa peripheral membrane protein of the trans-Golgi network. Owing to the presence of an N-terminal single baculovirus inhibitor repeat, BRUCE functions as an inhibitor of apoptosis protein and blocks apoptosis when overexpressed. In addition, due to the presence of a C-terminal ubiquitin-conjugating domain, BRUCE can covalently attach ubiquitin to substrates. Here we report the generation and characterization of BRUCE-deficient mice. Complete inactivation of the BRUCE gene resulted in perinatal lethality and growth retardation discernible after embryonic day 14. The growth defect is linked to impaired placental development and may be caused by insufficient oxygen and nutrient transfer across the placenta. Chorioallantoic placentation initiated normally, but the mutant placenta showed an impaired maturation of the labyrinth layer and a significant reduction of the spongiotrophoblast. No evidence for an elevated apoptosis rate was detectable in embryonic and extraembryonic tissues and in knockout fibroblasts. Thus, although BRUCE is broadly expressed in embryonic, extraembryonic, and adult mouse tissues, this bifunctional protein might play a unique role in normal trophoblast differentiation and embryonic survival.
Figures



Similar articles
-
Dual role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase.Mol Cell. 2004 Jun 18;14(6):801-11. doi: 10.1016/j.molcel.2004.05.018. Mol Cell. 2004. PMID: 15200957
-
Progressive loss of the spongiotrophoblast layer of Birc6/Bruce mutants results in embryonic lethality.Genesis. 2005 Jun;42(2):91-103. doi: 10.1002/gene.20128. Genesis. 2005. PMID: 15887267
-
Deletion of Pdcd5 in mice led to the deficiency of placenta development and embryonic lethality.Cell Death Dis. 2017 May 25;8(5):e2811. doi: 10.1038/cddis.2017.124. Cell Death Dis. 2017. PMID: 28542142 Free PMC article.
-
Regulation of apoptosis and cytokinesis by the anti-apoptotic E2/E3 ubiquitin-ligase BRUCE.Ernst Schering Found Symp Proc. 2008;(1):115-26. doi: 10.1007/2789_2008_104. Ernst Schering Found Symp Proc. 2008. PMID: 19198067 Review.
-
Research progress of E3 ubiquitin ligase regulating biological behavior of human placental trophoblast cells.Front Endocrinol (Lausanne). 2023 Apr 14;14:1124041. doi: 10.3389/fendo.2023.1124041. eCollection 2023. Front Endocrinol (Lausanne). 2023. PMID: 37168980 Free PMC article. Review.
Cited by
-
Regulation of cell death by the ubiquitin-proteasome system.Curr Opin Cell Biol. 2009 Dec;21(6):878-84. doi: 10.1016/j.ceb.2009.09.005. Epub 2009 Oct 21. Curr Opin Cell Biol. 2009. PMID: 19850458 Free PMC article. Review.
-
Suppressor mutations in Mecp2-null mice implicate the DNA damage response in Rett syndrome pathology.Genome Res. 2020 Apr;30(4):540-552. doi: 10.1101/gr.258400.119. Epub 2020 Apr 21. Genome Res. 2020. PMID: 32317254 Free PMC article.
-
Modulation of wolframin expression in human placenta during pregnancy: comparison among physiological and pathological states.Biomed Res Int. 2014;2014:985478. doi: 10.1155/2014/985478. Epub 2014 Jan 23. Biomed Res Int. 2014. PMID: 24588001 Free PMC article.
-
A potential role of X-linked inhibitor of apoptosis protein in mitochondrial membrane permeabilization and its implication in cancer therapy.Drug Discov Today. 2016 Jan;21(1):38-47. doi: 10.1016/j.drudis.2015.07.014. Epub 2015 Jul 30. Drug Discov Today. 2016. PMID: 26232549 Free PMC article. Review.
-
BRUCE preserves genomic stability in the male germline of mice.Cell Death Differ. 2020 Aug;27(8):2402-2416. doi: 10.1038/s41418-020-0513-4. Epub 2020 Mar 5. Cell Death Differ. 2020. PMID: 32139899 Free PMC article.
References
-
- Arama, E., J. Agapite, and H. Steller. 2003. Caspase activity and a specific cytochrome C are required for sperm differentiation in Drosophila. Dev. Cell 4:687-697. - PubMed
-
- Bartke, T., Pohl, C., G. Pyrowolakis, and S. Jentsch. 2004. Dual role of BRUCE as an antiapoptotic IAP and a chimeric E2/E3 ubiquitin ligase. Mol. Cell 14:801-811. - PubMed
-
- Chen, Z., M. Naito, S. Hori, T. Mashima, T. Yamori, and T. Tsuruo. 1999. A human IAP-family gene, apollon, expressed in human brain cancer cells. Biochem. Biophys. Res. Commun. 264:847-854. - PubMed
-
- Colosi, P., F. Talamantes, and D. I. Linzer. 1987. Molecular cloning and expression of mouse placental lactogen I complementary deoxyribonucleic acid. Mol. Endocrinol. 1:767-776. - PubMed
-
- Cross, J. C. 2000. Genetic insights into trophoblast differentiation and placental morphogenesis. Semin. Cell Dev. Biol. 11:105-113. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
