The cytomegalovirus m155 gene product subverts natural killer cell antiviral protection by disruption of H60-NKG2D interactions
- PMID: 15477345
- PMCID: PMC2211837
- DOI: 10.1084/jem.20040583
The cytomegalovirus m155 gene product subverts natural killer cell antiviral protection by disruption of H60-NKG2D interactions
Abstract
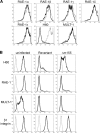
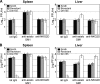
Natural killer (NK) cells are an important early mediator of host immunity to murine cytomegalovirus (MCMV) infection. However, MCMV has evolved mechanisms to elude recognition and clearance by NK cells. We have identified an MCMV immune evasion protein that impairs NKG2D-mediated NK cell antiviral activity. Infection of BALB/c 3T3 cells with the Smith strain of MCMV resulted in strong down-regulation of H60, a high affinity ligand for NKG2D, from the surface of virus-infected cells. The MCMV m155 protein specifically down-regulated H60 without affecting expression of the other known NKG2D ligands, RAE-1 and MULT-1. Treatment with the proteasome inhibitors lactacystin or epoxomicin reversed m155 down-regulation of H60. An MCMV mutant virus lacking m155 was severely attenuated in BALB/c mice; however, treatment with neutralizing anti-NKG2D monoclonal antibody or with NK-depleting anti-asialo GM1 antisera restored virulence of the mutant virus. Thus, down-regulation of H60 by m155 is a powerful mechanism of inhibiting NKG2D-mediated antiviral function.
Figures
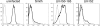

Similar articles
-
Selective down-regulation of the NKG2D ligand H60 by mouse cytomegalovirus m155 glycoprotein.J Virol. 2005 Mar;79(5):2920-30. doi: 10.1128/JVI.79.5.2920-2930.2005. J Virol. 2005. PMID: 15709011 Free PMC article.
-
NKG2D-mediated natural killer cell protection against cytomegalovirus is impaired by viral gp40 modulation of retinoic acid early inducible 1 gene molecules.J Exp Med. 2003 May 19;197(10):1245-53. doi: 10.1084/jem.20021973. J Exp Med. 2003. PMID: 12756263 Free PMC article.
-
Promiscuity of MCMV immunoevasin of NKG2D: m138/fcr-1 down-modulates RAE-1epsilon in addition to MULT-1 and H60.Mol Immunol. 2009 Nov;47(1):114-22. doi: 10.1016/j.molimm.2009.02.010. Epub 2009 Mar 17. Mol Immunol. 2009. PMID: 19297023
-
Murine cytomegalovirus regulation of NKG2D ligands.Med Microbiol Immunol. 2008 Jun;197(2):159-66. doi: 10.1007/s00430-008-0080-7. Epub 2008 Feb 8. Med Microbiol Immunol. 2008. PMID: 18259774 Review.
-
Lymphocyte activation via NKG2D: towards a new paradigm in immune recognition?Curr Opin Immunol. 2002 Jun;14(3):306-11. doi: 10.1016/s0952-7915(02)00337-0. Curr Opin Immunol. 2002. PMID: 11973127 Review.
Cited by
-
Structural basis for recognition of cellular and viral ligands by NK cell receptors.Front Immunol. 2014 Mar 26;5:123. doi: 10.3389/fimmu.2014.00123. eCollection 2014. Front Immunol. 2014. PMID: 24723923 Free PMC article. Review.
-
NK cell activation through the NKG2D ligand MULT-1 is selectively prevented by the glycoprotein encoded by mouse cytomegalovirus gene m145.J Exp Med. 2005 Jan 17;201(2):211-20. doi: 10.1084/jem.20041617. Epub 2005 Jan 10. J Exp Med. 2005. PMID: 15642742 Free PMC article.
-
Host-Adapted Gene Families Involved in Murine Cytomegalovirus Immune Evasion.Viruses. 2022 Jan 11;14(1):128. doi: 10.3390/v14010128. Viruses. 2022. PMID: 35062332 Free PMC article. Review.
-
Cytomegalovirus m154 hinders CD48 cell-surface expression and promotes viral escape from host natural killer cell control.PLoS Pathog. 2014 Mar 13;10(3):e1004000. doi: 10.1371/journal.ppat.1004000. eCollection 2014 Mar. PLoS Pathog. 2014. PMID: 24626474 Free PMC article.
-
NKG2D contributes to efficient clearance of picornavirus from the acutely infected murine brain.J Neurovirol. 2008 May;14(3):261-6. doi: 10.1080/13550280802105002. J Neurovirol. 2008. PMID: 18569460 Free PMC article.
References
-
- Reddehase, M.J. 2002. Antigens and immunoevasins: opponents in cytomegalovirus immune surveillance. Nat. Rev. Immunol. 2:831–844. - PubMed
-
- Tortorella, D., B.E. Gewurz, M.H. Furman, D.J. Schust, and H.L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861–926. - PubMed
-
- Biron, C.A., K.S. Byron, and J.L. Sullivan. 1989. Severe herpesvirus infections in an adolescent without natural killer cells. N. Engl. J. Med. 320:1731–1735. - PubMed
-
- Bauer, S., V. Groh, J. Wu, A. Steinle, J.H. Phillips, L.L. Lanier, and T. Spies. 1999. Activation of natural killer cells and T cells by NKG2D, a receptor for stress-inducible MICA. Science. 285:727–730. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

