Akt-1 expression level regulates CNS precursors
- PMID: 15456827
- PMCID: PMC6729906
- DOI: 10.1523/JNEUROSCI.1470-04.2004
Akt-1 expression level regulates CNS precursors
Abstract
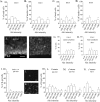
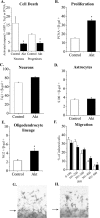
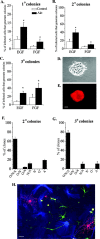
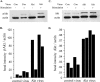
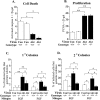
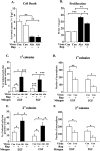
Although most cells in the embryonic mouse cortex express the serine-threonine kinase Akt-1, a small population of progenitors expresses Akt-1 protein at a higher level. To determine the functional significance of this difference, we used a retrovirus to increase Akt-1 expression in cortical progenitors. Increased Akt expression enhanced Akt activation after growth factor stimulation of progenitors. In vivo, it promoted retention in progenitor layers, the ventricular zone and subventricular zone. In vitro, it enhanced proliferation and survival, but did not impair migration. Moreover, it increased the proportion of stem cells, defined by a self-renewal assay. These effects did not depend on the Akt substrate p21(Cip1). In contrast, rapamycin, an inhibitor of mTOR (mammalian target of rapamycin), altered effects of elevated Akt-1 selectively: it eliminated the increase in stem cells and reduced the proliferative response, but had no effect on survival. The ability of elevated Akt-1 to increase the self-renewing population therefore depends on a rapamycin-sensitive mechanism (presumably inhibition of mTOR activity) but not on p21(Cip1), and can be distinguished from its effects on the proliferation and survival of other types of progenitors. Our findings suggest that expression of a high level of Akt-1 by a subpopulation of cortical progenitors biases their responses to extrinsic signals to increase their survival, proliferation, and/or self-renewal. Heterogeneity in Akt-1 level among progenitors could therefore allow cells that share a microenvironment to respond differently to the same extrinsic signals.
Figures

Similar articles
-
Role of the phosphatidylinositol 3-kinase/Akt and mTOR/P70S6-kinase pathways in the proliferation and apoptosis in multiple myeloma.Oncogene. 2002 Sep 26;21(43):6587-97. doi: 10.1038/sj.onc.1205923. Oncogene. 2002. PMID: 12242656
-
Roles of the Ras-MEK-mitogen-activated protein kinase and phosphatidylinositol 3-kinase-Akt-mTOR pathways in Jaagsiekte sheep retrovirus-induced transformation of rodent fibroblast and epithelial cell lines.J Virol. 2005 Apr;79(7):4440-50. doi: 10.1128/JVI.79.7.4440-4450.2005. J Virol. 2005. PMID: 15767444 Free PMC article.
-
Activation of the mammalian target of rapamycin pathway acutely inhibits insulin signaling to Akt and glucose transport in 3T3-L1 and human adipocytes.Endocrinology. 2005 Mar;146(3):1328-37. doi: 10.1210/en.2004-0777. Epub 2004 Dec 2. Endocrinology. 2005. PMID: 15576463
-
Activation of Akt and eIF4E survival pathways by rapamycin-mediated mammalian target of rapamycin inhibition.Cancer Res. 2005 Aug 15;65(16):7052-8. doi: 10.1158/0008-5472.CAN-05-0917. Cancer Res. 2005. PMID: 16103051
-
New inhibitors of the PI3K-Akt-mTOR pathway: insights into mTOR signaling from a new generation of Tor Kinase Domain Inhibitors (TORKinibs).Curr Top Microbiol Immunol. 2010;347:241-62. doi: 10.1007/82_2010_64. Curr Top Microbiol Immunol. 2010. PMID: 20549474 Review.
Cited by
-
Electroacupuncture Facilitates the Integration of Neural Stem Cell-Derived Neural Network with Transected Rat Spinal Cord.Stem Cell Reports. 2019 Feb 12;12(2):274-289. doi: 10.1016/j.stemcr.2018.12.015. Epub 2019 Jan 17. Stem Cell Reports. 2019. PMID: 30661994 Free PMC article.
-
Transient silencing of PTEN in human CD34(+) cells enhances their proliferative potential and ability to engraft immunodeficient mice.Exp Hematol. 2012 Jan;40(1):84-91. doi: 10.1016/j.exphem.2011.10.001. Epub 2011 Oct 20. Exp Hematol. 2012. PMID: 22019626 Free PMC article.
-
Selective induction of neocortical GABAergic neurons by the PDK1-Akt pathway through activation of Mash1.Proc Natl Acad Sci U S A. 2009 Aug 4;106(31):13064-9. doi: 10.1073/pnas.0808400106. Epub 2009 Jun 19. Proc Natl Acad Sci U S A. 2009. PMID: 19549840 Free PMC article.
-
β-aminoisobutyric acid attenuates LPS-induced inflammation and insulin resistance in adipocytes through AMPK-mediated pathway.J Biomed Sci. 2018 Mar 28;25(1):27. doi: 10.1186/s12929-018-0431-7. J Biomed Sci. 2018. PMID: 29592806 Free PMC article.
-
Neurodevelopmental effects of insulin-like growth factor signaling.Front Neuroendocrinol. 2012 Aug;33(3):230-51. doi: 10.1016/j.yfrne.2012.06.002. Epub 2012 Jun 16. Front Neuroendocrinol. 2012. PMID: 22710100 Free PMC article. Review.
References
-
- Alessi DR, Caudwell FB, Andjelkovic M, Hemmings BA, Cohen P (1996b) Molecular basis for the substrate specificity of protein kinase B; comparison with MAPKAP kinase-1 and p70 S6 kinase. FEBS Lett 399: 333-338. - PubMed
-
- Brazil DP, Hemmings BA (2001) Ten years of protein kinase B signalling: a hard Akt to follow. Trends Biochem Sci 26: 657-664. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
