Detection of snRNP assembly intermediates in Cajal bodies by fluorescence resonance energy transfer
- PMID: 15452143
- PMCID: PMC2172029
- DOI: 10.1083/jcb.200405160
Detection of snRNP assembly intermediates in Cajal bodies by fluorescence resonance energy transfer
Abstract
Spliceosomal small nuclear ribonucleoprotein particles (snRNPs) are required for pre-mRNA splicing throughout the nucleoplasm, yet snRNPs also concentrate in Cajal bodies (CBs). To address a proposed role of CBs in snRNP assembly, we have used fluorescence resonance energy transfer (FRET) microscopy to investigate the subnuclear distribution of specific snRNP intermediates. Two distinct complexes containing the protein SART3 (p110), required for U4/U6 snRNP assembly, were localized: SART3.U6 snRNP and SART3.U4/U6 snRNP. These complexes segregated to different nuclear compartments, with SART3.U6 snRNPs exclusively in the nucleoplasm and SART3.U4/U6 snRNPs preferentially in CBs. Mutant cells lacking the CB-specific protein coilin and consequently lacking CBs exhibited increased nucleoplasmic levels of SART3.U4/U6 snRNP complexes. Reconstitution of CBs in these cells by expression of exogenous coilin restored accumulation of SART3.U4/U6 snRNP in CBs. Thus, while some U4/U6 snRNP assembly can occur in the nucleoplasm, these data provide evidence that SART3.U6 snRNPs form in the nucleoplasm and translocate to CBs where U4/U6 snRNP assembly occurs.
Figures
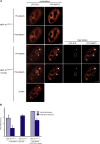
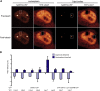
Similar articles
-
Targeting of U4/U6 small nuclear RNP assembly factor SART3/p110 to Cajal bodies.J Cell Biol. 2003 Feb 17;160(4):505-16. doi: 10.1083/jcb.200210087. Epub 2003 Feb 10. J Cell Biol. 2003. PMID: 12578909 Free PMC article.
-
RNAi knockdown of hPrp31 leads to an accumulation of U4/U6 di-snRNPs in Cajal bodies.EMBO J. 2004 Aug 4;23(15):3000-9. doi: 10.1038/sj.emboj.7600296. Epub 2004 Jul 15. EMBO J. 2004. PMID: 15257298 Free PMC article.
-
In vivo kinetics of U4/U6·U5 tri-snRNP formation in Cajal bodies.Mol Biol Cell. 2011 Feb 15;22(4):513-23. doi: 10.1091/mbc.E10-07-0560. Epub 2010 Dec 22. Mol Biol Cell. 2011. PMID: 21177826 Free PMC article.
-
The Cajal body: a meeting place for spliceosomal snRNPs in the nuclear maze.Chromosoma. 2006 Oct;115(5):343-54. doi: 10.1007/s00412-006-0056-6. Epub 2006 Mar 31. Chromosoma. 2006. PMID: 16575476 Review.
-
The assembly of a spliceosomal small nuclear ribonucleoprotein particle.Nucleic Acids Res. 2008 Nov;36(20):6482-93. doi: 10.1093/nar/gkn658. Epub 2008 Oct 14. Nucleic Acids Res. 2008. PMID: 18854356 Free PMC article. Review.
Cited by
-
Cajal bodies and snRNPs - friends with benefits.RNA Biol. 2017 Jun 3;14(6):671-679. doi: 10.1080/15476286.2016.1231359. Epub 2016 Sep 14. RNA Biol. 2017. PMID: 27627834 Free PMC article. Review.
-
A distant coilin homologue is required for the formation of cajal bodies in Arabidopsis.Mol Biol Cell. 2006 Jul;17(7):2942-51. doi: 10.1091/mbc.e05-12-1157. Epub 2006 Apr 19. Mol Biol Cell. 2006. PMID: 16624863 Free PMC article.
-
On the origin of non-membrane-bound organelles, and their physiological function.J Theor Biol. 2017 Dec 7;434:42-49. doi: 10.1016/j.jtbi.2017.04.006. Epub 2017 Apr 6. J Theor Biol. 2017. PMID: 28392184 Free PMC article.
-
The Drosophila melanogaster Cajal body.J Cell Biol. 2006 Mar 13;172(6):875-84. doi: 10.1083/jcb.200511038. J Cell Biol. 2006. PMID: 16533947 Free PMC article.
-
Periodic expression of Sm proteins parallels formation of nuclear Cajal bodies and cytoplasmic snRNP-rich bodies.Histochem Cell Biol. 2011 Nov;136(5):527-41. doi: 10.1007/s00418-011-0861-8. Epub 2011 Sep 9. Histochem Cell Biol. 2011. PMID: 21904826 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

