PKC controls HGF-dependent c-Met traffic, signalling and cell migration
- PMID: 15385963
- PMCID: PMC522795
- DOI: 10.1038/sj.emboj.7600396
PKC controls HGF-dependent c-Met traffic, signalling and cell migration
Abstract
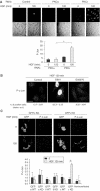
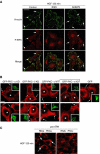
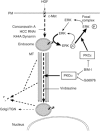
The growth factor/receptor pair HGF/c-Met exerts control on proliferation, morphogenesis and motility, and through overexpression and mutation is implicated in cancer. Here we have investigated the relationship between receptor signalling and traffic, and its control by specific PKC isotypes. It is shown that c-Met signalling to the ERK cascade occurs within endosomal compartments and that it is in this compartment that PKCepsilon specifically exerts its control on the pathway with the consequent accumulation of ERK in focal complexes. These events are clearly separated from the subsequent microtubule-dependent sorting of c-Met to its perinuclear destination, which is shown to be under the control of PKCalpha. Thus while it is shown that traffic to endosomes is essential for HGF/c-Met to trigger an ERK response, the subsequent traffic and signalling of c-Met controlled by these two PKC isotypes are unconnected events. The dynamic properties conferred by the PKCepsilon control are shown to be essential for a normal HGF-dependent migratory response. Thus PKCs are shown to control both receptor traffic and signal traffic to relay HGF/c-Met responses.
Figures



Similar articles
-
Mechanisms of hepatocyte growth factor-induced retinal endothelial cell migration and growth.Invest Ophthalmol Vis Sci. 2000 Jun;41(7):1885-93. Invest Ophthalmol Vis Sci. 2000. PMID: 10845613
-
PKC mediates fluctuant ERK-paxillin signaling for hepatocyte growth factor-induced migration of hepatoma cell HepG2.Cell Signal. 2013 Jun;25(6):1457-67. doi: 10.1016/j.cellsig.2013.03.011. Epub 2013 Mar 22. Cell Signal. 2013. PMID: 23524339
-
MAP kinase and beta-catenin signaling in HGF induced RPE migration.Mol Vis. 2002 Dec 20;8:483-93. Mol Vis. 2002. PMID: 12500177
-
Structure, biosynthesis and biochemical properties of the HGF receptor in normal and malignant cells.EXS. 1993;65:131-65. EXS. 1993. PMID: 8380735 Review.
-
c-Met signalling: spatio-temporal decisions.Cell Cycle. 2005 Mar;4(3):352-5. doi: 10.4161/cc.4.3.1519. Epub 2005 Mar 7. Cell Cycle. 2005. PMID: 15701970 Review.
Cited by
-
B cell linker protein (BLNK) is a regulator of Met receptor signaling and trafficking in non-small cell lung cancer.iScience. 2022 Oct 20;25(11):105419. doi: 10.1016/j.isci.2022.105419. eCollection 2022 Nov 18. iScience. 2022. PMID: 36388990 Free PMC article.
-
Targeting Protein Kinase C Downstream of Growth Factor and Adhesion Signalling.Cancers (Basel). 2015 Jul 15;7(3):1271-91. doi: 10.3390/cancers7030836. Cancers (Basel). 2015. PMID: 26184315 Free PMC article. Review.
-
Inhibition of IFN-alpha signaling by a PKC- and protein tyrosine phosphatase SHP-2-dependent pathway.Proc Natl Acad Sci U S A. 2005 Jul 19;102(29):10267-72. doi: 10.1073/pnas.0408854102. Epub 2005 Jul 6. Proc Natl Acad Sci U S A. 2005. PMID: 16000408 Free PMC article.
-
c-Met and Other Cell Surface Molecules: Interaction, Activation and Functional Consequences.Biomedicines. 2015 Jan 15;3(1):46-70. doi: 10.3390/biomedicines3010046. Biomedicines. 2015. PMID: 28536399 Free PMC article. Review.
-
Identification of an oncogenic RAB protein.Science. 2015 Oct 9;350(6257):211-7. doi: 10.1126/science.aaa4903. Epub 2015 Sep 3. Science. 2015. PMID: 26338797 Free PMC article.
References
-
- Alves dos Santos CM, van Kerkhof P, Strous GJ (2001) The signal transduction of the growth hormone receptor is regulated ubiquitin/proteasome system and continues after endocytosis. J Biol Chem 276: 10839–10846 - PubMed
-
- Awasthi V, King RJ (2000) PKC, p42/p44 MAPK, and p38 MAPK are required for HGF-induced of H441 cells. Am J Physiol Lung Cell Mol Physiol 279: L942–L949 - PubMed
-
- Chapell R, Bueno OF, Alvarez-Hernandez X, Robinson LC, Leidenheimer NJ (1998) Activation of protein kinase C induces gamma-aminobutyric acid type A receptor internalization in Xenopus oocytes. J Biol Chem 273: 32595–32601 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

