Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis
- PMID: 15381762
- PMCID: PMC521973
- DOI: 10.1073/pnas.0406216101
Responsive transporter genes within the murine intestinal-pancreatic axis form a basis of zinc homeostasis
Abstract
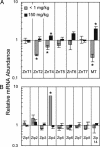
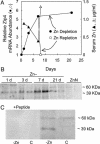
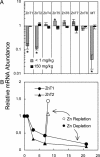
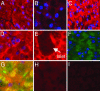
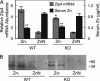
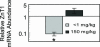
Zn homeostasis in animals is a consequence of avid uptake and retention, except during conditions of limited dietary availability, and/or factors such as parasites, which compete for this micronutrient or compromise retention by the host. Membrane proteins that facilitate Zn transport constitute the SLC30A (ZnT) and SLC39A (Zip) gene families. Because dietary recommendations are based on the balance between intestinal absorption and endogenous losses, we have studied Zn transporter expression of the murine intestinal-pancreatic axis to identify transporters that are likely to be involved in homeostatic control of Zn metabolism. Marked tissue specificity of expression was observed in Zn-depleted vs. Zn-adequate mice. As shown by quantitative PCR, Western blot analysis, and immunohistochemistry, intestinal Zip4 was markedly up-regulated in response to Zn-depletion conditions. The increased abundance of Zip4 is concentrated at the apical membrane of enterocytes. There are 16 ZnT and Zip transporters expressed in pancreas. Only two, ZnT1 and ZnT2 (both cellular Zn exporters), show a progressive down-regulation under Zn-depleted conditions. In Zn-adequate mice, ZnT1 is diffusely distributed in acinar cell cytoplasm and colocalizes with alpha-amylase but is not detected in pancreatic islets. In acinar cells during Zn depletion, ZnT1 is localized to the plasma membrane. Intestinal Zip4 up-regulation by Zn-depletion conditions is dampened in metallothionein knockout mice, suggesting that intracellular Zn pools influence these responses. The results show that Zn transporter expression in the intestinal-pancreatic axis is a component of the homeostatic regulation of this micronutrient.
Figures


Similar articles
-
Tissue-specific alterations in zinc transporter expression in intestine and liver reflect a threshold for homeostatic compensation during dietary zinc deficiency in weanling rats.J Nutr. 2009 May;139(5):835-41. doi: 10.3945/jn.108.100974. Epub 2009 Mar 18. J Nutr. 2009. PMID: 19297427
-
Increased expression of zinc transporter ZIP4, ZIP11, ZnT1, and ZnT6 predicts poor prognosis in pancreatic cancer.J Trace Elem Med Biol. 2021 May;65:126734. doi: 10.1016/j.jtemb.2021.126734. Epub 2021 Feb 17. J Trace Elem Med Biol. 2021. PMID: 33631610
-
Gastrointestinal factors influencing zinc absorption and homeostasis.Int J Vitam Nutr Res. 2010 Oct;80(4-5):243-8. doi: 10.1024/0300-9831/a000030. Int J Vitam Nutr Res. 2010. PMID: 21462106 Free PMC article. Review.
-
A high amount of dietary zinc changes the expression of zinc transporters and metallothionein in jejunal epithelial cells in vitro and in vivo but does not prevent zinc accumulation in jejunal tissue of piglets.J Nutr. 2013 Aug;143(8):1205-10. doi: 10.3945/jn.113.177881. Epub 2013 Jun 12. J Nutr. 2013. PMID: 23761649
-
Mammalian zinc transporters: nutritional and physiologic regulation.Annu Rev Nutr. 2009;29:153-76. doi: 10.1146/annurev-nutr-033009-083312. Annu Rev Nutr. 2009. PMID: 19400752 Review.
Cited by
-
Zinc supplementation of young men alters metallothionein, zinc transporter, and cytokine gene expression in leukocyte populations.Proc Natl Acad Sci U S A. 2006 Feb 7;103(6):1699-704. doi: 10.1073/pnas.0510407103. Epub 2006 Jan 24. Proc Natl Acad Sci U S A. 2006. PMID: 16434472 Free PMC article. Clinical Trial.
-
Zinc transporter ZIP8 (SLC39A8) and zinc influence IFN-gamma expression in activated human T cells.J Leukoc Biol. 2009 Aug;86(2):337-48. doi: 10.1189/jlb.1208759. Epub 2009 Apr 28. J Leukoc Biol. 2009. PMID: 19401385 Free PMC article.
-
Effect of zinc intake on hepatic autophagy during acute alcohol intoxication.Biometals. 2018 Apr;31(2):217-232. doi: 10.1007/s10534-018-0077-7. Epub 2018 Feb 1. Biometals. 2018. PMID: 29392448 Free PMC article.
-
Association of cadmium with diabetes in middle-aged residents of abandoned metal mines: the first health effect surveillance for residents in abandoned metal mines.Ann Occup Environ Med. 2015 Aug 24;27:20. doi: 10.1186/s40557-015-0071-2. eCollection 2015. Ann Occup Environ Med. 2015. PMID: 26306202 Free PMC article.
-
A novel epigenetic CREB-miR-373 axis mediates ZIP4-induced pancreatic cancer growth.EMBO Mol Med. 2013 Sep;5(9):1322-34. doi: 10.1002/emmm.201302507. Epub 2013 Jul 16. EMBO Mol Med. 2013. PMID: 23857777 Free PMC article.
References
-
- Liuzzi, J. P. & Cousins, R. J. (2004) Annu. Rev. Nutr. 24, 151-172. - PubMed
-
- Wastney, M. E., House, W. A., Barnes, R. M. & Subramanian, K. N. (2000) J. Nutr. 130, 1355S-1359S. - PubMed
-
- Panel on Micronutrients, Subcommittees on Upper Reference Levels of Nutrients and of Interpretation and Use of Dietary Reference Intakes, and the Standing Committee on the Scientific Evaluation of Dietary Reference Intakes (2002) in Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc, eds. Food and Nutrition Board (Natl. Acad. Press, Washington, DC), pp. 442-501.
-
- Hoadley, J. E., Leinart, A. S. & Cousins, R. J. (1987) Am. J. Physiol. 252, G825-G831. - PubMed
-
- Cousins, R. J. (1996) in Present Knowledge in Nutrition, eds. Filer, L. J. & Ziegler, E. E. (ILSI, Washington, DC), 7th Ed., pp. 293-306.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

