CD4 T cells producing IFN-gamma in the lungs of mice challenged with mycobacteria express a CD27-negative phenotype
- PMID: 15373901
- PMCID: PMC1809176
- DOI: 10.1111/j.1365-2249.2004.02573.x
CD4 T cells producing IFN-gamma in the lungs of mice challenged with mycobacteria express a CD27-negative phenotype
Abstract
Protection against tuberculosis depends upon the generation of CD4(+) T cell effectors capable of producing IFN-gamma and stimulating macrophage antimycobacterial function. Effector CD4(+) T cells are known to express CD44(hi)CD62L(lo) surface phenotype. In this paper we demonstrate that a population of CD44(hi)CD62L(lo) CD4(+) effectors generated in response to Mycobacterium bovis BCG or M. tuberculosis infection in C57BL/6 mice is heterogeneous and consists of CD27(hi) and CD27(lo) T cell subsets. These subsets exhibit a similar degree of in vivo proliferation, but differ by the capacity for IFN-gamma production. Ex vivo isolated CD27(lo) T cells express higher amounts of IFN-gamma RNA and contain higher frequencies of IFN-gamma producers compared to CD27(hi) subset, as shown by real-time PCR, intracellular staining for IFN-gamma and ELISPOT assays. In addition, CD27(lo) CD4(+) T cells uniformly express CD44(hi)CD62L(lo) phenotype. We propose that CD27(lo) CD44(hi)CD62L(lo) CD4(+) T cells represent highly differentiated effector cells with a high capacity for IFN-gamma secretion and antimycobacterial protection at the site of infection.
Figures
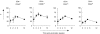


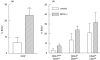
 ) mice were pulsed with BrdU at week 2 postinfection. 24 h later lung cells were stained with FITC-anti-BrdU, PE-anti-CD27, PerCP-anti-CD4 and APC-anti-CD44 mAbs and analysed by Flow cytometry. (a) Percent of BrdU+ cells within CD4+ subset of lung cells. (b) Percent of BrdU+ cells within subsets of CD4+ T cells with different expression of CD44 and CD27 molecules. Data are mean ± SD from 2 independent experiments (total of 6 mice per group analysed individually). *P < 0·05; **P < 0·01; as compared to control mice.
) mice were pulsed with BrdU at week 2 postinfection. 24 h later lung cells were stained with FITC-anti-BrdU, PE-anti-CD27, PerCP-anti-CD4 and APC-anti-CD44 mAbs and analysed by Flow cytometry. (a) Percent of BrdU+ cells within CD4+ subset of lung cells. (b) Percent of BrdU+ cells within subsets of CD4+ T cells with different expression of CD44 and CD27 molecules. Data are mean ± SD from 2 independent experiments (total of 6 mice per group analysed individually). *P < 0·05; **P < 0·01; as compared to control mice.
Similar articles
-
Prime-boost vaccination strategy with bacillus Calmette-Guérin (BCG) and liposomized alpha-crystalline protein 1 reinvigorates BCG potency.Clin Exp Immunol. 2015 Aug;181(2):286-96. doi: 10.1111/cei.12634. Epub 2015 Jun 3. Clin Exp Immunol. 2015. PMID: 25845290 Free PMC article.
-
Mice deficient in CD4 T cells have only transiently diminished levels of IFN-gamma, yet succumb to tuberculosis.J Immunol. 1999 May 1;162(9):5407-16. J Immunol. 1999. PMID: 10228018
-
CD27low CD4 T lymphocytes that accumulate in the mouse lungs during mycobacterial infection differentiate from CD27high precursors in situ, produce IFN-gamma, and protect the host against tuberculosis infection.J Immunol. 2007 Jan 15;178(2):976-85. doi: 10.4049/jimmunol.178.2.976. J Immunol. 2007. PMID: 17202360
-
Trying to See the Forest through the Trees: Deciphering the Nature of Memory Immunity to Mycobacterium tuberculosis.Front Immunol. 2018 Mar 8;9:461. doi: 10.3389/fimmu.2018.00461. eCollection 2018. Front Immunol. 2018. PMID: 29568298 Free PMC article. Review.
-
The macrophage in tuberculosis: sinner or saint? The T cell decides.Pathobiology. 1991;59(3):153-5. doi: 10.1159/000163634. Pathobiology. 1991. PMID: 1883509 Review.
Cited by
-
Systemic BCG immunization induces persistent lung mucosal multifunctional CD4 T(EM) cells which expand following virulent mycobacterial challenge.PLoS One. 2011;6(6):e21566. doi: 10.1371/journal.pone.0021566. Epub 2011 Jun 24. PLoS One. 2011. PMID: 21720558 Free PMC article.
-
Targeting B-cell malignancies through human B-cell receptor specific CD4+ T cells.Oncoimmunology. 2016 Sep 19;5(11):e1232220. doi: 10.1080/2162402X.2016.1232220. eCollection 2016. Oncoimmunology. 2016. PMID: 27999743 Free PMC article.
-
Mitochondrial Transplantation Promotes Protective Effector and Memory CD4+ T Cell Response During Mycobacterium Tuberculosis Infection and Diminishes Exhaustion and Senescence in Elderly CD4+ T cells.Adv Sci (Weinh). 2024 Sep;11(36):e2401077. doi: 10.1002/advs.202401077. Epub 2024 Jul 22. Adv Sci (Weinh). 2024. PMID: 39039808 Free PMC article.
-
Loss of receptor on tuberculin-reactive T-cells marks active pulmonary tuberculosis.PLoS One. 2007 Aug 15;2(8):e735. doi: 10.1371/journal.pone.0000735. PLoS One. 2007. PMID: 17710135 Free PMC article.
-
Pulmonary Mycobacterium bovis BCG vaccination confers dose-dependent superior protection compared to that of subcutaneous vaccination.Clin Vaccine Immunol. 2014 Apr;21(4):594-7. doi: 10.1128/CVI.00700-13. Epub 2014 Feb 5. Clin Vaccine Immunol. 2014. PMID: 24501340 Free PMC article.
References
-
- Colditz GA, Brewer TF, Berkey CS, Wilson ME, Burdick E, Fineberg HV, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis. Meta-analysis of the published literature. J Am Med Assoc. 1981;271:698–702. - PubMed
-
- North RJ. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973;7:166–76. - PubMed
-
- D’Souza CD, Cooper AM, Frank AA, Mazzaccaro RJ, Bloom BR, Orme IM. An. anti-inflammatory role for gamma delta T lymphocytes in acquired immunity to Mycobacterium tuberculosis. J Immunol. 1997;158:1217–21. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

