Integration of clinical data, pathology, and cDNA microarrays in influenza virus-infected pigtailed macaques (Macaca nemestrina)
- PMID: 15367608
- PMCID: PMC516400
- DOI: 10.1128/JVI.78.19.10420-10432.2004
Integration of clinical data, pathology, and cDNA microarrays in influenza virus-infected pigtailed macaques (Macaca nemestrina)
Abstract
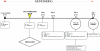
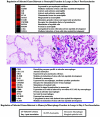
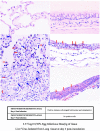
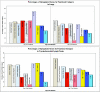
For most severe viral pandemics such as influenza and AIDS, the exact contribution of individual viral genes to pathogenicity is still largely unknown. A necessary step toward that understanding is a systematic comparison of different influenza virus strains at the level of transcriptional regulation in the host as a whole and interpretation of these complex genetic changes in the context of multifactorial clinical outcomes and pathology. We conducted a study by infecting pigtailed macaques (Macaca nemestrina) with a genetically reconstructed strain of human influenza H1N1 A/Texas/36/91 virus and hypothesized not only that these animals would respond to the virus similarly to humans, but that gene expression patterns in the lungs and tracheobronchial lymph nodes would fit into a coherent and complete picture of the host-virus interactions during infection. The disease observed in infected macaques simulated uncomplicated influenza in humans. Clinical signs and an antibody response appeared with induction of interferon and B-cell activation pathways, respectively. Transcriptional activation of inflammatory cells and apoptotic pathways coincided with gross and histopathological signs of inflammation, with tissue damage and concurrent signs of repair. Additionally, cDNA microarrays offered new evidence of the importance of cytotoxic T cells and natural killer cells throughout infection. With this experiment, we confirmed the suitability of the nonhuman primate model in the quest for understanding the individual and joint contributions of viral genes to influenza virus pathogenesis by using cDNA microarray technology and a reverse genetics approach.
Figures




Similar articles
-
Integrated molecular signature of disease: analysis of influenza virus-infected macaques through functional genomics and proteomics.J Virol. 2006 Nov;80(21):10813-28. doi: 10.1128/JVI.00851-06. Epub 2006 Aug 23. J Virol. 2006. PMID: 16928763 Free PMC article.
-
Dynamic gene expression analysis in a H1N1 influenza virus mouse pneumonia model.Virus Genes. 2017 Jun;53(3):357-366. doi: 10.1007/s11262-017-1438-y. Epub 2017 Feb 27. Virus Genes. 2017. PMID: 28243843
-
Virus-specific antigen presentation by different subsets of cells from lung and mediastinal lymph node tissues of influenza virus-infected mice.J Virol. 1995 Oct;69(10):6359-66. doi: 10.1128/JVI.69.10.6359-6366.1995. J Virol. 1995. PMID: 7666537 Free PMC article.
-
Use of functional genomics to understand influenza-host interactions.Adv Virus Res. 2007;70:81-100. doi: 10.1016/S0065-3527(07)70003-9. Adv Virus Res. 2007. PMID: 17765704 Free PMC article. Review.
-
Contribution of innate immune cells to pathogenesis of severe influenza virus infection.Clin Sci (Lond). 2017 Feb 1;131(4):269-283. doi: 10.1042/CS20160484. Clin Sci (Lond). 2017. PMID: 28108632 Review.
Cited by
-
Animal Models for Influenza Research: Strengths and Weaknesses.Viruses. 2021 May 28;13(6):1011. doi: 10.3390/v13061011. Viruses. 2021. PMID: 34071367 Free PMC article. Review.
-
Deoxycytidyl-deoxyguanosine oligonucleotide classes A, B, and C induce distinct cytokine gene expression patterns in rhesus monkey peripheral blood mononuclear cells and distinct alpha interferon responses in TLR9-expressing rhesus monkey plasmacytoid dendritic cells.Clin Diagn Lab Immunol. 2005 May;12(5):606-21. doi: 10.1128/CDLI.12.5.606-621.2005. Clin Diagn Lab Immunol. 2005. PMID: 15879022 Free PMC article.
-
Old world monkeys and new age science: the evolution of nonhuman primate systems virology.ILAR J. 2013;54(2):166-80. doi: 10.1093/ilar/ilt039. ILAR J. 2013. PMID: 24174440 Free PMC article. Review.
-
Extrapulmonary tissue responses in cynomolgus macaques (Macaca fascicularis) infected with highly pathogenic avian influenza A (H5N1) virus.Arch Virol. 2010 Jun;155(6):905-14. doi: 10.1007/s00705-010-0662-8. Epub 2010 Apr 7. Arch Virol. 2010. PMID: 20372944 Free PMC article.
-
Proteomics of Bronchoalveolar Lavage Fluid Reveals a Lung Oxidative Stress Response in Murine Herpesvirus-68 Infection.Viruses. 2018 Nov 27;10(12):670. doi: 10.3390/v10120670. Viruses. 2018. PMID: 30486363 Free PMC article.
References
-
- Abed, Y., A. M. Bourgault, R. J. Fenton, P. J. Morley, D. Gower, I. J. Owens, M. Tisdale, and G. Boivin. 2002. Characterization of 2 influenza A(H3N2) clinical isolates with reduced susceptibility to neuraminidase inhibitors due to mutations in the hemagglutinin gene. J. Infect. Dis. 186:1074-1080. - PubMed
-
- Achdout, H., T. I. Arnon, G. Markel, T. Gonen-Gross, G. Katz, N. Lieberman, R. Gazit, A. Joseph, E. Kedar, and O. Mandelboim. 2003. Enhanced recognition of human NK receptors after influenza virus infection. J. Immunol. 171:915-923. - PubMed
-
- Ada, G. L., and P. D. Jones. 1986. The immune response to influenza infection. Curr. Top. Microbiol. Immunol. 128:1-54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

