Severe defects in dorsal thalamic development in low-density lipoprotein receptor-related protein-6 mutants
- PMID: 15342729
- PMCID: PMC6729615
- DOI: 10.1523/JNEUROSCI.2123-04.2004
Severe defects in dorsal thalamic development in low-density lipoprotein receptor-related protein-6 mutants
Abstract
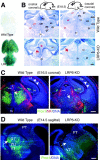
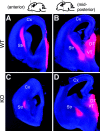
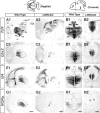
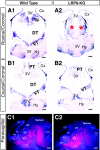
Mice with mutations in the Wnt coreceptor low-density lipoprotein receptor-related protein-6 (LRP6) have a smaller and severely disorganized dorsal thalamus and lack thalamocortical projections. Using molecular markers, we showed that most dorsal thalamic and epithalamic neurons were missing, and most of the major dorsal thalamic nuclei were not identifiable. However, the ventral thalamus was essentially unaffected, although the dorsal thalamic defect leads to rostral displacement of portions of the ventral thalamus. Analysis of younger embryos showed that epithalamic and dorsal thalamic neurons were not produced at early stages of development, whereas ventral thalamic neurons were still produced. These defects were accompanied by improper formation of the boundary between dorsal and ventral thalamus, the zona limitans interthalamica (ZLI). Furthermore, the expression of an early marker of posterior forebrain development that marks the compartment from the midbrain-hindbrain junction to the ZLI (including the future dorsal thalamus, pretectum, and midbrain) was disrupted, supporting the idea that diencephalic development is abnormal from very early in embryogenesis. This study provides compelling in vivo evidence that thalamic development requires normal activity of the LRP6-mediated canonical Wnt signaling pathway.
Figures


Similar articles
-
A role for Pax6 in the normal development of dorsal thalamus and its cortical connections.Development. 2000 Dec;127(23):5167-78. doi: 10.1242/dev.127.23.5167. Development. 2000. PMID: 11060242
-
The mechanism of endogenous receptor activation functionally distinguishes prototype canonical and noncanonical Wnts.Mol Cell Biol. 2005 May;25(9):3475-82. doi: 10.1128/MCB.25.9.3475-3482.2005. Mol Cell Biol. 2005. PMID: 15831454 Free PMC article.
-
Hedgehog signalling from the zona limitans intrathalamica orchestrates patterning of the zebrafish diencephalon.Development. 2006 Mar;133(5):855-64. doi: 10.1242/dev.02248. Epub 2006 Feb 1. Development. 2006. PMID: 16452095
-
Wnt signaling and osteoblastogenesis.Rev Endocr Metab Disord. 2006 Jun;7(1-2):33-9. doi: 10.1007/s11154-006-9002-4. Rev Endocr Metab Disord. 2006. PMID: 16960757 Review.
-
Heads or tails? Amphioxus and the evolution of anterior-posterior patterning in deuterostomes.Dev Biol. 2002 Jan 15;241(2):209-28. doi: 10.1006/dbio.2001.0503. Dev Biol. 2002. PMID: 11784106 Review.
Cited by
-
Postnatal isoform switch and protein localization of LEF1 and TCF7L2 transcription factors in cortical, thalamic, and mesencephalic regions of the adult mouse brain.Brain Struct Funct. 2013 Nov;218(6):1531-49. doi: 10.1007/s00429-012-0474-6. Epub 2012 Nov 15. Brain Struct Funct. 2013. PMID: 23152144 Free PMC article.
-
Building a bridal chamber: development of the thalamus.Trends Neurosci. 2010 Aug;33(8):373-80. doi: 10.1016/j.tins.2010.05.003. Epub 2010 Jun 11. Trends Neurosci. 2010. PMID: 20541814 Free PMC article. Review.
-
Differential gene expression in the developing lateral geniculate nucleus and medial geniculate nucleus reveals novel roles for Zic4 and Foxp2 in visual and auditory pathway development.J Neurosci. 2009 Oct 28;29(43):13672-83. doi: 10.1523/JNEUROSCI.2127-09.2009. J Neurosci. 2009. PMID: 19864579 Free PMC article.
-
Complex and dynamic patterns of Wnt pathway gene expression in the developing chick forebrain.Neural Dev. 2009 Sep 4;4:35. doi: 10.1186/1749-8104-4-35. Neural Dev. 2009. PMID: 19732418 Free PMC article.
-
Patterning and compartment formation in the diencephalon.Front Neurosci. 2012 May 11;6:66. doi: 10.3389/fnins.2012.00066. eCollection 2012. Front Neurosci. 2012. PMID: 22593732 Free PMC article.
References
-
- Bagri A, Gurney T, He X, Zou YR, Littman DR, Tessier-Lavigne M, Pleasure SJ (2002) The chemokine SDF1 regulates migration of dentate granule cells. Development 129: 4249-4260. - PubMed
-
- Bramblett DE, Copeland NG, Jenkins NA, Tsai MJ (2002) BHLHB4 is a bHLH transcriptional regulator in pancreas and brain that marks the dimesencephalic boundary. Genomics 79: 402-412. - PubMed
-
- Braun MM, Etheridge A, Bernard A, Robertson CP, Roelink H (2003) Wnt signaling is required at distinct stages of development for the induction of the posterior forebrain. Development 130: 5579-5587. - PubMed
-
- Caspary T, Anderson KV (2003) Patterning cell types in the dorsal spinal cord: what the mouse mutants say. Nat Rev Neurosci 4: 289-297. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
