Two novel proteins in the mitochondrial outer membrane mediate beta-barrel protein assembly
- PMID: 15326197
- PMCID: PMC2172422
- DOI: 10.1083/jcb.200405138
Two novel proteins in the mitochondrial outer membrane mediate beta-barrel protein assembly
Abstract
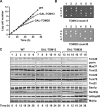
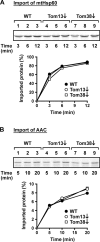
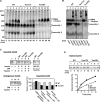
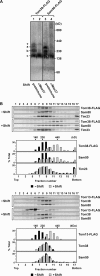
Mitochondrial outer and inner membranes contain translocators that achieve protein translocation across and/or insertion into the membranes. Recent evidence has shown that mitochondrial beta-barrel protein assembly in the outer membrane requires specific translocator proteins in addition to the components of the general translocator complex in the outer membrane, the TOM40 complex. Here we report two novel mitochondrial outer membrane proteins in yeast, Tom13 and Tom38/Sam35, that mediate assembly of mitochondrial beta-barrel proteins, Tom40, and/or porin in the outer membrane. Depletion of Tom13 or Tom38/Sam35 affects assembly pathways of the beta-barrel proteins differently, suggesting that they mediate different steps of the complex assembly processes of beta-barrel proteins in the outer membrane.
Figures

Similar articles
-
Machinery for protein sorting and assembly in the mitochondrial outer membrane.Nature. 2003 Jul 31;424(6948):565-71. doi: 10.1038/nature01753. Nature. 2003. PMID: 12891361
-
The morphology proteins Mdm12/Mmm1 function in the major beta-barrel assembly pathway of mitochondria.EMBO J. 2007 May 2;26(9):2229-39. doi: 10.1038/sj.emboj.7601673. Epub 2007 Apr 5. EMBO J. 2007. PMID: 17410204 Free PMC article.
-
Integral membrane proteins in the mitochondrial outer membrane of Saccharomyces cerevisiae.FEBS J. 2006 Apr;273(7):1507-15. doi: 10.1111/j.1742-4658.2006.05171.x. FEBS J. 2006. PMID: 16689936
-
Biogenesis of cytochrome oxidase-sophisticated assembly lines in the mitochondrial inner membrane.Gene. 2005 Jul 18;354:43-52. doi: 10.1016/j.gene.2005.03.017. Gene. 2005. PMID: 15905047 Review.
-
How does the TOM complex mediate insertion of precursor proteins into the mitochondrial outer membrane?J Cell Biol. 2005 Nov 7;171(3):419-23. doi: 10.1083/jcb.200507147. Epub 2005 Oct 31. J Cell Biol. 2005. PMID: 16260501 Free PMC article. Review.
Cited by
-
Mim1, a protein required for the assembly of the TOM complex of mitochondria.EMBO Rep. 2005 Jan;6(1):57-62. doi: 10.1038/sj.embor.7400318. EMBO Rep. 2005. PMID: 15608614 Free PMC article.
-
Precursor binding to an 880-kDa Toc complex as an early step during active import of protein into chloroplasts.Plant J. 2007 Jan;49(1):149-58. doi: 10.1111/j.1365-313X.2006.02944.x. Epub 2006 Nov 28. Plant J. 2007. PMID: 17144891 Free PMC article.
-
Roles of the Mdm10, Tom7, Mdm12, and Mmm1 proteins in the assembly of mitochondrial outer membrane proteins in Neurospora crassa.Mol Biol Cell. 2010 May 15;21(10):1725-36. doi: 10.1091/mbc.e09-10-0844. Epub 2010 Mar 24. Mol Biol Cell. 2010. PMID: 20335503 Free PMC article.
-
Signals in bacterial beta-barrel proteins are functional in eukaryotic cells for targeting to and assembly in mitochondria.Proc Natl Acad Sci U S A. 2009 Feb 24;106(8):2531-6. doi: 10.1073/pnas.0807830106. Epub 2009 Jan 30. Proc Natl Acad Sci U S A. 2009. PMID: 19181862 Free PMC article.
-
Multiple pathways for sorting mitochondrial precursor proteins.EMBO Rep. 2008 Jan;9(1):42-9. doi: 10.1038/sj.embor.7401126. EMBO Rep. 2008. PMID: 18174896 Free PMC article. Review.
References
-
- Endo, T., H. Yamamoto, and M. Esaki. 2003. Functional cooperation and separation of translocators in protein import into mitochondria, the double-membrane bounded organelles. J. Cell Sci. 116:3259–3267. - PubMed
-
- Dekker, P.J.T., F. Martin, A.C. Maarse, U. Bömer, H. Müller, B. Guiard, M. Meijer, J. Rassow, and N. Pfanner. 1997. The Tim core complex defines the number of mitochondrial translocation contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 16:5408–5419. - PMC - PubMed
-
- Herrmann, J.M., and W. Neupert. 2000. Protein import into mitochondria. Curr. Opin. Microbiol. 3:210–214. - PubMed
-
- Hill, K., K. Model, M.T. Ryan, K. Dietmeier, F. Martin, R. Wagner, and N. Pfanner. 1998. Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature. 395:516–521. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

